Hafnium(Iv) Carbide
 From Handwiki
From Handwiki 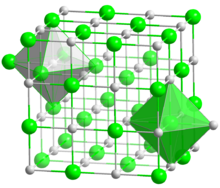
| |
| Identifiers | |
|---|---|
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
HfC |
| Molar mass | 190.50 g/mol |
| Appearance | black odorless powder |
| Density | 12.2 g/cm3[1] |
| Melting point | 3,900 °C (7,050 °F; 4,170 K)[2] |
Solubility in water
|
insoluble |
| Structure | |
Crystal structure
|
Cubic crystal system, cF8 |
Space group
|
Fm3m, No. 225 |
| Hazards | |
EU classification (DSD) (outdated)
|
not listed |
| NFPA 704 (fire diamond) | 
2
2
1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Hafnium carbide (HfC) is a chemical compound of hafnium and carbon. With a melting point of about 3900 °C it is one of the most refractory binary compounds known.[2] However, it has a low oxidation resistance, with the oxidation starting at temperatures as low as 430 °C.[3] This compound may be seen on future spacecraft as part of the heat shield.
Hafnium carbide is usually carbon deficient and therefore its composition is often expressed as HfCx (x = 0.5 to 1.0). It has a cubic (rock-salt) crystal structure at any value of x.[4]
Hafnium carbide powder is obtained by the reduction of hafnium(IV) oxide with carbon at 1800 to 2000 °C. A long processing time is required to remove all oxygen. Alternatively, high-purity HfC coatings can be obtained by chemical vapor deposition from a gas mixture of methane, hydrogen, and vaporized hafnium(IV) chloride. Because of the technical complexity and high cost of the synthesis, HfC has a very limited use, despite its favorable properties such as high hardness (>9 Mohs[5]) and melting point.[2]
The magnetic properties of HfCx change from paramagnetic for x ≤ 0.8 to diamagnetic at larger x. An inverse behavior (dia-paramagnetic transition with increasing x) is observed for TaCx, despite its having the same crystal structure as HfCx.[6]
References
- ↑ Physical Constants of Inorganic Compounds in Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. pp. 4–44 ff.. ISBN 0-8493-0486-5.
- ↑ 2.0 2.1 2.2 Harry Julius Emeléus (1968). Advances in Inorganic Chemistry and Radiochemistry. Academic Press. pp. 169–170. ISBN 978-0-12-023611-4. https://books.google.com/books?id=-SnCsg5jM_kC&pg=PA169.
- ↑ Shimada, Shiro (1992). "Oxidation Kinetics of Hafnium Carbide in the Temperature Range of 480o to 600oC". Journal of the American Ceramic Society 75 (10): 2671–2678. doi:10.1111/j.1151-2916.1992.tb05487.x.
- ↑ Lavrentyev, A; Gabrelian, B; Vorzhev, V; Nikiforov, I; Khyzhun, O; Rehr, J (2008). "Electronic structure of cubic HfxTa1–xCy carbides from X-ray spectroscopy studies and cluster self-consistent calculations". Journal of Alloys and Compounds 462 (1–2): 4–10. doi:10.1016/j.jallcom.2007.08.018.
- ↑ CRC Materials Science and Engineering Handbook (2001).
- ↑ Aleksandr Ivanovich Gusev; Andreĭ Andreevich Rempel; Andreas J. Magerl (2001). Disorder and order in strongly nonstoichiometric compounds: transition metal carbides, nitrides, and oxides. Springer. pp. 513–516. ISBN 978-3-540-41817-7. https://books.google.com/books?id=jc2D7TGZcyUC&pg=PA513.
Categories: [Carbides] [Hafnium compounds]
↧ Download as ZWI file | Last modified: 05/11/2023 04:26:45 | 3 views
☰ Source: https://handwiki.org/wiki/Chemistry:Hafnium(IV)_carbide | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF