Selenium Tetrabromide
 From Handwiki
From Handwiki 
| |
| |
| Names | |
|---|---|
| IUPAC name
Tetrabromo-λ4-selane
| |
| Other names
Selenium tetrabromide, selenium(IV) bromide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
SeBr4 |
| Molar mass | 398.576 |
| Density | 4.029 g/cm3 |
| Melting point | 75 °C (167 °F; 348 K) (dissolves) |
| Boiling point | 115 °C (239 °F; 388 K) (sublimes) |
| Structure[1] | |
Crystal structure
|
trigonal (α) monoclinic (β) |
Space group
|
P31c, No. 159 (α) C2/c, No.15 (β) |
Formula units (Z)
|
16 |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
GHS hazard statements
|
H301, H311, H314, H331, H351, H373, H410 |
GHS precautionary statements
|
P201, P202, P260, P261, P264, P270, P271, P273, P280, P281, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P308+313, P310, P311, P312, P314, P321, P322, P330, P361 |
| Related compounds | |
Other anions
|
Selenium tetrafluoride Selenium tetrachloride |
Other cations
|
Tellurium tetrabromide |
Related compounds
|
Selenium dibromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Selenium tetrabromide is an inorganic compound with a chemical formula SeBr4.
Preparation
Selenium tetrabromide could be produced by mixing elemental bromine and selenium:[2][3]
- [math]\displaystyle{ \rm \ Se + 2Br_2 \rightarrow SeBr_4 }[/math]
Properties
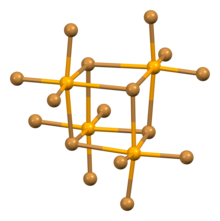
Selenium tetrabromide exists in two polymorphs, the trigonal, black α-SeBr4 and the monoclinic, orange-reddish β-SeBr4, both of which feature tetrameric cubane-like Se4Br16 units but differ in how they are arranged.[1] It dissolves in carbon disulfide, chloroform and ethyl bromide, but decomposes in water,[4] so that it produces selenous acid in wet air.
The compound is only stable under a bromine-saturated atmosphere; gas phase measurements of the gas density indicate that the compound decomposes into selenium monobromide and bromine.[3]
- [math]\displaystyle{ \rm \ 2SeBr_4 \rightarrow Se_2Br_2 + 3Br_2 }[/math]
References
- ↑ 1.0 1.1 Born, Ref. P.; Kniep, R.; Mootz, D. (1979). "Phasenbeziehungen im System Se-Br und die Kristallstrukturen des dimorphen SeBr4". Z. Anorg. Allg. Chem. 451 (1): 12–24. doi:10.1002/zaac.19794510103. https://onlinelibrary.wiley.com/doi/abs/10.1002/zaac.19794510103.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. pp. 772-774. ISBN 978-0-08-037941-8.
- ↑ 3.0 3.1 Tideswell, N. W.; McCullough, J. D. (1956). "Selenium Bromides. I. A Spectrophotometric Study of the Dissociation of Selenium Tetrabromide and Selenium Dibromide in Carbon Tetrachloride Solution1,2". Journal of the American Chemical Society 78 (13): 3026–3029. doi:10.1021/ja01594a025.
- ↑ Perry, Dale L. (2011). Handbook of Inorganic Compounds. CRC Press. pp. 360. ISBN 978-1-4398-1461-1.
 |
Categories: [Bromides]
↧ Download as ZWI file | Last modified: 11/11/2024 15:04:03 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Selenium_tetrabromide | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF