Acetone
 From Conservapedia
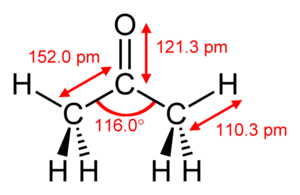
From Conservapedia Acetone (CH3COCH3, sometimes called "Dimethyl Ketone"[1]) is an organic substance which is a type of Ketone molecule, often used as a solvent and sometimes also as a disinfectant. It is a colorless liquid which can dissolve in water, is flammable, and toxic in high quantities. Among other potential heath impacts, it is known to effect Hematological (Blood Forming) and Neurological (Nervous System) organ systems in the human body. For these reasons, it is recognized as a "Volatile organic compound."[2] In lesser quantities, it is generally a harmless but unneeded bi-product of fat breakdown and other natural processes.[3]
Uses[edit]
As a caustic substance, this is often used to dissolve fingernail polish and other cosmetics, varnish, ink, fats, resins, and a variety of ethers. Acetone is also frequently used in the manufacture of a variety of synthetic products, including plastics, rayon, and drugs.[1]
References[edit]
- ↑ 1.0 1.1 "acetone (CH3COCH3)." Encyclopaedia Britannica. Britannica Academic. Encyclopædia Britannica Inc., 2016. Web. 12 May. 2016. <http://0-academic.eb.com.www.consuls.org/EBchecked/topic/3270/acetone>.
- ↑ http://www.atsdr.cdc.gov/substances/toxsubstance.asp?toxid=1
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/acetone
Categories: [Organic Chemistry]
↧ Download as ZWI file | Last modified: 02/08/2023 19:57:37 | 18 views
☰ Source: https://www.conservapedia.com/Acetone | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: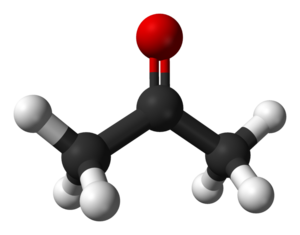
 KSF
KSF