Neodymium(Iii) Iodide
 From Handwiki
From Handwiki | Names | |
|---|---|
| IUPAC name
Triiodoneodymium
| |
| Other names
Neodymium triiodide, Neodymium iodide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
NdI3 |
| Molar mass | 524.96 g/mol |
| Appearance | Green solid |
| Melting point | 684 °C (1,263 °F; 957 K) |
| Structure | |
Coordination geometry
|
9 |
| Hazards | |
| GHS pictograms |    [1] [1]
|
| GHS Signal word | Danger[2] |
| Related compounds | |
Other anions
|
Neodymium acetate, Neodymium hydride, Neodymium nickelate |
Other cations
|
erbium iodide, cerium iodide, terbium iodide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Neodymium(III) iodide is an inorganic salt of iodine and neodymium with the formula NdI3.[3] Neodymium uses the +3 oxidation state in the compound. The anhydrous compound[2] is a green powdery[2] solid at room temperature.
Preparation
Heating neodymium and iodine in an inert atmosphere produces this salt:[citation needed]
- 2 Nd + 3 I2 → 2 NdI3
It can also be prepared by the reaction of neodymium(III) oxide and hydroiodic acid to make a hydrate:[4]
- Nd2O3 + 6 HI → 2 NdI3 + 3 H2O
The anhydrate can then be obtained by heating the nonahydrate with ammonium iodide:[4]
- NdI3 • 9 H2O + nNH4I → NdI3 + nNH3 + nHI + 9H2O
Physical properties
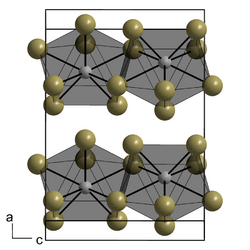
Neodymium(III) iodide forms green, water-soluble hygroscopic crystals. It has a melting point of 784°C. It forms a nonahydrate crystal NdI3.9H2O – belongs to the orthorhombic crystal system, space group Pmmn, lattice constants a = 1.16604 nm, b = 0.80103 nm, c = 0.89702 nm, Z = 4.[5]
Other compounds
NdI3 also forms some compounds with N2H4, such as NdI3·3N2H4·2H2O which is a dark green crystal, soluble in methanol and ethanol and insoluble in water, benzene and toluene, d20°C = 3.42 g/cm³.[6]
NdI3 also forms some compounds with urea, such as NdI3 5CO(NH2)2 which is a lavender color crystal.[7]
NdI3 also forms some compounds with thiourea, such as NdI3·2CS(NH2)2·9H2O which is a pale pink crystal.[8]
See also
- Neodymium(II) iodide
- Neodymium
- Iodine
- Lanthanide
References
- ↑ See https://onyxmet.com/index.php?route=product/product&product_id=2733
- ↑ 2.0 2.1 2.2 See https://pubchem.ncbi.nlm.nih.gov/compound/Neodymium-triiodide#datasheet=LCSS
- ↑ Ezhov, Y.S., Komarov, S.A. & Sevast’yanov, V.G. Refinement of molecular constants of neodymium triiodide by electron diffraction. J Struct Chem 41, 593–596 (2000). https://doi.org/10.1007/BF02683920
- ↑ 4.0 4.1 Kutscher, J.; Schneider, A. (1971-09-01). "Notiz zur Präparation von wasserfreien Lanthaniden-Haloge-niden, Insbesondere von Jodiden" (in de). Inorganic and Nuclear Chemistry Letters 7 (9): 815–819. doi:10.1016/0020-1650(71)80253-2. ISSN 0020-1650. https://dx.doi.org/10.1016/0020-1650(71)80253-2.
- ↑ T. Timofte, A. Babai, G. Meyer and A.-V. Mudring (2005). "Neodymium triiodide nonahydrate". Acta Crystallographica Section E E61 (5): i87–i88. doi:10.1107/S160053680501216X.
- ↑ Uchenye zapiski: Serii︠a︡ khimicheskikh nauk (S.M. Kirov adyna Azărbai̐jan Dȯvlăt Universiteti; 1975), page 78. Retrieved January 13, 2021. (Translated from Vietnamese)
- ↑ Russian Journal of Inorganic Chemistry, Episode 18, Part 2 (British Library Lending Division with the cooperation of the Royal Society of Chemistry, 1973), page 1655. Retrieved 13 January 2021.
- ↑ Alikberova L.Yu., Albov D.V., Antonenko T.A., Kochetova I.M., Rukk N.S. — Thiourea complexes of neodymium(III) and gadolinium(III) iodides. synthesis and structure. Fine Chemical Technologies. 2010, 5 (3): 30–33. (in Russian).
| HI | He | ||||||||||||||||
| LiI | BeI2 | BI3 | CI4 | NI3 | I2O4, I2O5, I4O9 |
IF, IF3, IF5, IF7 |
Ne | ||||||||||
| NaI | MgI2 | AlI3 | SiI4 | PI3, P2I4 |
S | ICl, ICl3 |
Ar | ||||||||||
| KI | CaI2 | Sc | TiI4 | VI3 | CrI3 | MnI2 | FeI2 | CoI2 | NiI2 | CuI | ZnI2 | Ga2I6 | GeI2, GeI4 |
AsI3 | Se | IBr | Kr |
| RbI | SrI2 | YI3 | ZrI4 | NbI5 | Mo | Tc | Ru | Rh | Pd | AgI | CdI2 | InI3 | SnI4, SnI2 |
SbI3 | TeI4 | I | Xe |
| CsI | BaI2 | HfI4 | TaI5 | W | Re | Os | Ir | Pt | AuI | Hg2I2, HgI2 |
TlI | PbI2 | BiI3 | Po | AtI | Rn | |
| Fr | RaI2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La | Ce | Pr | Nd | Pm | SmI2 | Eu | Gd | TbI3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac | ThI4 | Pa | UI3, UI4 |
Np | Pu | Am | Cm | Bk | Cf | EsI3 | Fm | Md | No | Lr | |||
 |
Categories: [Neodymium(III) compounds] [Iodides]
↧ Download as ZWI file | Last modified: 08/26/2025 09:01:20 | 9 views
☰ Source: https://handwiki.org/wiki/Chemistry:Neodymium(III)_iodide | License: CC BY-SA 3.0

 KSF
KSF