Foraminifera
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Foraminifera, in zoology, a subdivision of Protozoa, the name selected for this enormous class being that given by A. D’Orbigny in 1826 to the shells characteristic of the majority of the species. He regarded them as minute Cephalopods, whose chambers communicated by pores (foramina). Later on their true nature was discovered by F. Dujardin, working on living forms, and he referred them to his Rhizopoda, characterized by pseudopodia given off from the sarcode (protoplasm) as organs of prehension and locomotion. W.B. Carpenter in 1862 differentiated the group nearly in its present limits as “Reticularia”; and since then it has been rendered more natural by the removal of a number of simple forms (mostly freshwater) with branching but not reticulate pseudopods, to Filosa, a distinct subclass, now united with Lobosa into the restricted class of Rhizopoda.
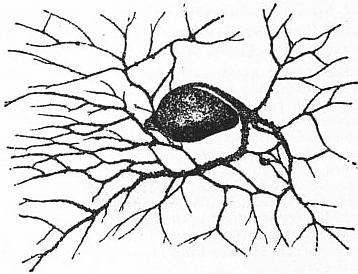 |
| Fig. 1A.—Lieberkühnia, with reticulate pseudopodia. |
Anatomy.—Protista Sarcodina, with simple protoplasmic bodies of granular surface, emitting processes which branch and anastomose freely, either from the whole surface or from one or more elongated processes (“stylopods”); nucleus one or more (not yet demonstrated in some little known simple forms), usually in genetic relation to granules or strands of matter of similar composition, the “chromidia” scattered through the protoplasm; body naked, or provided with a permanent investment (shell or test), membranous, gelatinous, arenaceous (of compacted or cemented granules), calcareous, or very rarely (in deep sea forms) siliceous, sometimes freely perforated, but never latticed; opening by one or more permanent apertures (“pylomes”) or crevices between compacted sand-granules, often very complex; reproduction by fission (only in simplest naked forms), or by brood formation; in the latter case one mode of brood formation (A) eventuates in amoebiform embryos, the other (B) in flagellate zoospores which are exogamous gametes, pairing but not with those of their own brood; the coupled cell (“zygote”) when mature in the shelled species gives rise to a very small primitive test-chamber or “microsphere.” The adult microspheric animal gives rise to the amoebiform brood which have a larger primitive test (“megalosphere”); and megalospheric forms appear to reproduce by the A type a series of similar forms before a B brood of gametes is finally borne, to pair and reproduce the microspheric type, which is consequently rare.
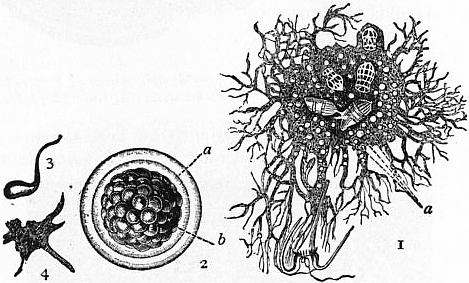 | |
| Fig. 1B.—Protomyxa aurantiaca, Haeck. (After Haeckel.) | |
|
1, Adult, containing two diatom frustules, and three Tintinnid ciliates, with a large Dinoflagellate just caught by the expanded reticulate pseudopodia. |
2, Adult encysted and segmented. 3, Flagellate zoospore just freed from cyst. 4, Zoospore which has passed into the amoeboid state. |
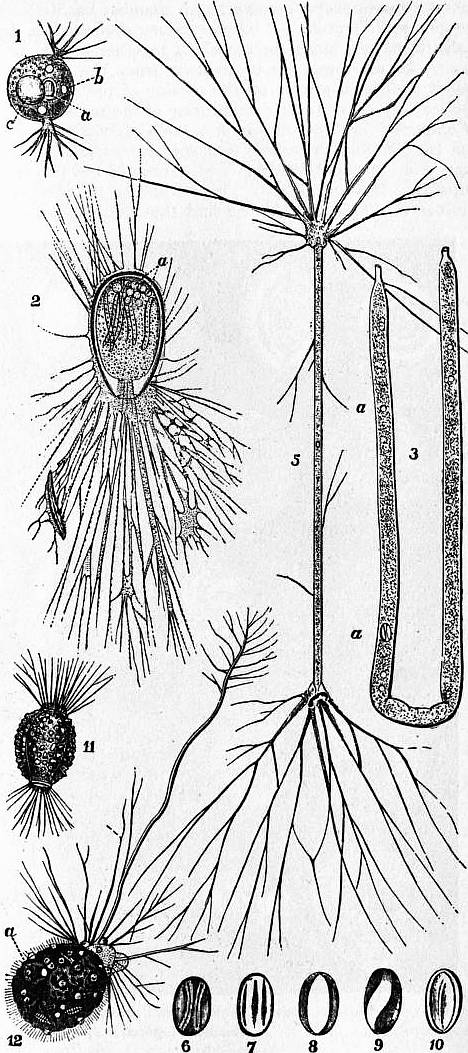 | |
| Fig. 2.—Allogromiidea. | |
|
1, Diplophrys archeri, Barker. 2, Allogromia oviformis, Duj. 3, Shepheardella taeniiformis,
Siddall (Quart. Jour. Micr.
Sci., 1880). |
5, Shepheardella taeniiformis; with pseudopodia fully expanded. 6-10, Varying appearance of the nucleus as it is carried along in the streaming protoplasm within the tube. 11, Amphitrema wrightianum, Archer, showing membranous shell encrusted with foreign particles. Moor pools, Ireland. 12, Diaphorophodon mobile,
Archer. |
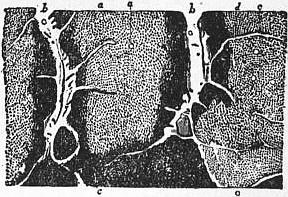
The shells require special study. In the lowest forms they are membranous, sometimes encrusted with sand-grains, always very simple, the only complication being the doubling of the pylome in Diplophrys (fig. 2, 1), Shepheardella (fig. 2, 3-5), Amphitrema (fig. 2, 11), Diaphorophodon (fig. 2, 12). The marine shells are, as we have seen, of cemented particles, or calcareous, glassy, and regularly perforated, or again calcareous, but porcellanous and rarely perforate. These characters have been used as a guide to classification; but some sandy forms have so large a proportion of calcareous cement that they might well be called encrusted calcareous genera, and are also not very constant in respect of the character of perforation. The porcellanous genera, however, form a compact group, the replacement of the shell by silica in forms dwelling in the red clay of the ocean abysses, where calcium carbonate is soluble, not really making any difficulty. Moreover, the shells of this group show a deflected process or neck of the embryonic chamber (“camptopyle”) at least in the megalospheric forms, whereas when such a neck exists in other groups it is straight. The opening of the shell is called the pylome. This may be a mere hole where the lateral walls of the body end, or there may be a diaphragmatic ingrowth so as to narrow the entrance. It may be a simple rounded opening, oblong or tri-multi-radiate, or branching (fig. 4, 1); or replaced by a number of coarse pores (“ethmopyle”) (fig. 3, 5a). Again, it may lie at the end of a narrowed tube (“stylopyle”), which in Lagena (fig. 3, 9) may project outwards (“ectoselenial”), or inwards (“entoselenial”). In most groups the stylopyle is straight; but in the majority of the porcellanous shells it is bent down on the side of the shell, and constitutes the “flexopyle” of A. Kemna, which being a hybrid term should be replaced by “camptopyle.” The animal usually forms a simple shell only after it has attained a certain size, and this “embryonic chamber” cannot grow further. In Spirillina and Ammodiscus there is no pylomic end-wall, and the shell continues to grow as a spiral tube; in Cornuspira (fig. 3, 1) there is a slight constriction indicating the junction of a small embryonic chamber with a camptopyle, but the rest of the shell is a simple flat spiral of several turns. In the majority at least one chamber follows the first, with its own pylome at the distal end. This second chamber may rest on the first, so that the part on which it rests serves as a party-wall bounding the front of the newer chamber as well as the back of the older; and this state prevails for all added chambers in such cases. In the highest vitreous shells, however, each chamber has its complete “proper wall”; while a “supplementary skeleton,” a deposit of shelly matter, binds the chambers together into a compact whole. In all cases the protoplasm from the pylome may deposit additional matter on the outside of the shell, so as to produce very characteristic sculpturing of the surface.
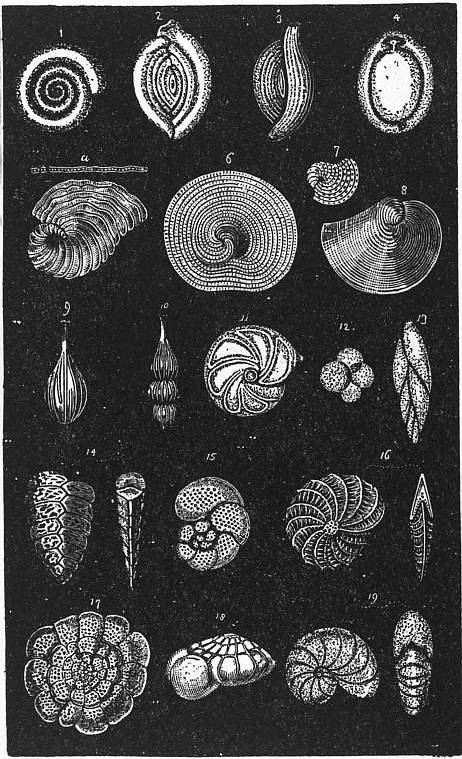 | ||
| Fig. 3.—Various forms of Calcareous Foraminifera. | ||
1, Cornuspira. 2, Spiroloculina. 3, Triloculina. 4, Biloculina. 5, Peneroplis. 6, Orbiculina (cyclical). 7, Orbiculina (young). |
8, Orbiculina (spiral). 9, Lagena. 10, Nodosaria. 11, Cristellaria. 12, Globigerina. 13, Polymorphina. |
14, Textularia. 15, Discorbina. 16, Polystomella. 17, Planorbulina. 18, Rotalia. 19, Nonionina. |
 |
| Fig. 4.—Modifications of Peneroplis. 1, Dendritina; 2, Eu-Peneroplis. |
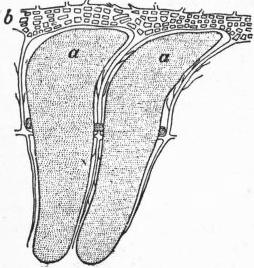
Compound or “polythalamic” shells derive their general form largely from the relations of successive chambers in size, shape and direction. This is well shown in the porcellanous Miliolidae. If we call the straight line uniting the two ends of a chamber the “polar axis,” we find that successive chambers have their pylomes at alternate poles; but they lie on different meridians. In Spiroloculina (fig. 3, 2) the divergence between the meridians is 180°, and the chambers are strongly incurved, so that the whole shell forms a flat spiral, of nearly circular outline. In the majority, however, the chambers are crescentic in section, their transverse prolongations being termed “alary” outgrowths, so that successive chambers overlap; when under this condition the angle of successive meridians is still 180° we have the form Biloculina (fig. 3, 4), or with the alary extensions completely enveloping, Uniloculina; when the angle is 120° we have Triloculina, or 144°, Quinqueloculina. Again in Peneroplis (figs. 3, 5, and 4) the shell begins as a flattened shell which tends to straighten out with further growth and additional chambers. In some forms (Spirolina, fig. 22, 3) the chambers have a nearly circular transverse section, and the adult shell is thus crozier-shaped. In others (which may have the same sculpture, and are scarcely distinguishable as species) the chambers are short and wide, drawn out at right angles to the axis, but in the plane of the spiral, and the growing shell becomes fan-shaped or “flabelliform” (figs. 3, 5, 4, 2). This widening may go on till the outer chambers form the greater part of a circle, as in Orbiculina (fig. 3, 6-8) where, moreover, each large chamber is subdivided by incomplete vertical bulkheads into a tier of chamberlets; each chamberlet has a distinct pylomic pore opening to the outside or to those of the next outer zone. In Orbitolites (figs. 5, 6) we have a centre on a somewhat Milioline type; and after a few chambers in spiral succession, complete circles of chambers are formed. In the larger forms the new zones are of greater height, and horizontal bulkheads divide the chamberlets into vertical tiers, each with its own pylomic pore.
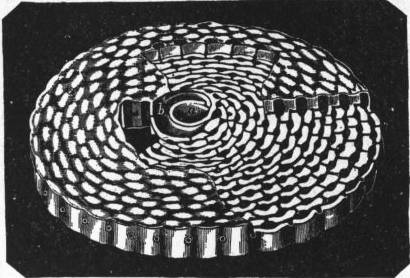 |
| Fig. 5.—Shell of simple type of Orbitolites, showing primordial chamber a, and circumambient chamber b, surrounded by successive rings of chamberlets connected by circular galleries which open at the margin by pores. |
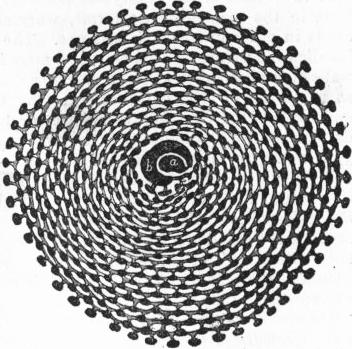 |
| Fig. 6.—Animal of simple type of Orbitolites, showing primordial segment a, and circumambient segment b, surrounded by annuli of sub-segments connected by radial and circular stolon-processes. |
The Cheilostomellidae (fig. 3, 13) reproduce among perforate vitreous genera what we have already seen in the Miliolida: Orbitoides (fig. 10, 2) and Cycloclypeus, among the Nummulite group, with a very finely perforate wall, recall the porcellanous Orbiculina and Orbitolites.
In flat spiral forms (figs. 22, 1, 7; 3, 2, 16, 19, &c.) all the chambers may be freely exposed; or the successive chambers be wider transversely than their predecessors and overlap by “alary extensions,” becoming “nautiloid”; in extreme cases only the last turn or whorl is seen (fig. 11). When the spiral axis is conical the shell may be “rotaloid,” the larger lower chambers partially concealing the upper smaller ones (fig. 3, 12, 15, 17, 18); or they may leave, as in Patellina, a wide central conical cavity—which, in this genus, is finally occupied by later formed “supplementary” chambers. When the successive chambers are disposed around a longitudinal central axis they may be said to “alternate” like the leaves of a plant. If the arrangement is distichous we get such forms as Polymorphina, Textularia and Frondicularia (fig. 3, 13, 14), if tristichous, Tritaxia. Such an arrangement may coexist with a spiral twist of the axis for at least part of its course, as in the crozier-shaped Spiroplecta.
 |
| Fig. 7.—Section of Rotalia beccarii, showing the canal system, a, b, c, in the substance of the intermediate skeleton; d, tubulated chamber-wall. |
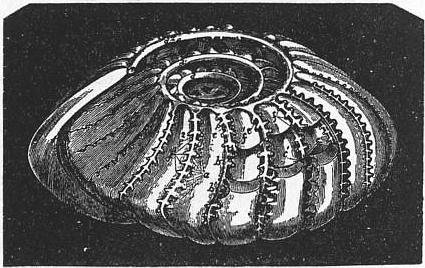 | |
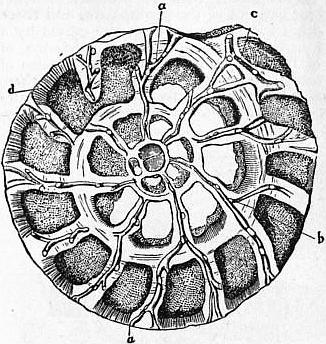
| Fig. 8.—Internal cast of Polystomella craticulata. | |
a, Retral processes, proceeding from the posterior margin of one of the segments. b, b¹, Smooth anterior margin of the same segment. c, c¹, Stolons connecting successive segments and uniting themselves with the diverging branches of the meridional canals. |
d, d¹, d², Three turns of one of the spiral canals. e, e¹, e², Three of the meridional canals. f, f¹, f², Their diverging branches. |
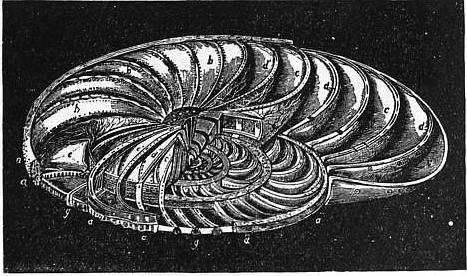 | |
| Fig. 9.—Operculina laid open, to show its internal structure. | |
a, Marginal cord seen in cross section at a’. b, b, External walls of the chambers. c, c, Cavities of the chambers. c′, c′, Their alar prolongations. |
d, d, Septa divided at d’, d’, and at d”, so as to lay open the interseptal canals, the general distribution of which is seen in the septa e, e; the lines radiating from e, e point to the secondary pores. g, g, Non-tubular columns. |
Two phenomena interfere with the ready availability of the characters of form for classificatory ends—dimorphism and multiformity.
Dimorphism.—The majority of foraminiferal shells show two types, the rarer with a much smaller central chamber than that of the more frequent. The chambers are called microsphere and megalosphere, the forms in which they occur microsphaeric and megalosphaeric forms, respectively. We shall study below their relation to the reproductive cycle.
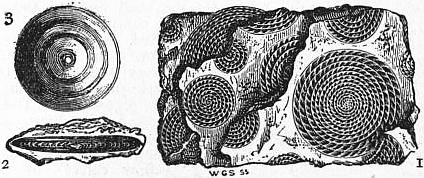 |
| Fig. 10.—1, Piece of Nummulitic Limestone from the Pyrenees, showing Nummulites laid open by fracture through the median plane; 2, vertical section of Nummulite; 3, Orbitoides. |
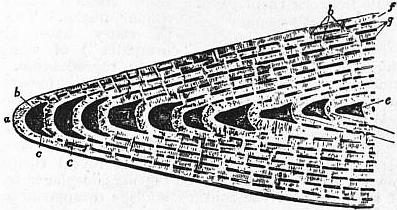 | |
| Fig. 11.—Vertical section of portion of Nummulites, showing the investment of the earlier whorls by the alar prolongations of the later. | |
a, Marginal cord. b, Chamber of outer whorl. c, c, Whorl invested by a. d, One of the chambers of the fourth whorl from the margin. e, e′, Marginal portions of the enclosed whorls. |
f, Investing portion of the outer whorl. g, g, Spaces left between the investing portions of successive whorls. h, h, Sections of the partitions dividing these. |
 |
| Fig. 12.—Internal surface of wall of two chambers, a, a, of Nummulites, showing the orifices of its minute tubuli. |
b, b, The septa containing canals. c, c, Extensions of these canals in the intermediate skeleton. d, d, Larger pores. |
Multiformity.—Many of the Polythalamia show different types of chamber-succession at different ages. We have noted this phenomenon in such crozier forms as Peneroplis, as well as in discoid forms; it is very frequent. Thus the microspheric Biloculina form the first few chambers in quinqueloculine succession. The microspheric forms attain to a greater size when adult than the megalospheric; and in Orbitolites the microsphere has a straight outlet, orthostyle, instead of the deflected camptostyle one, so general in porcellanous types; and the spiral succession is continued for more turns before reaching the fan-shaped and finally cyclic stage. Globigerina, whose chambers are nearly spherical, is sometimes seen to be enclosed in a spherical test, perforate, but without a pylome, and known as Orbulina; the chambered Globigerina-shell is attached at first inside the wall of the Orbulina, but ultimately disappears. The ultimate fate of the Orbulina shell is unknown; but it obviously marks a turning-point in the life-cycle.
 |
| Fig. 13.—Internal cast of two chambers, a, a, of Nummulites, the radial canals between them passing into b, marginal plexus. |
Protoplasmic Body and Reproduction.—The protoplasm is not differentiated into ecto- and endosarc, although it is often denser in the central part within the shell, and clearer in the pseudopodial ramifications and the layer (or stalk in the monothalamic forms) from which it is given off. In pelagic forms like Globigerina the external layer is almost if not quite identical in structure with the extracapsular protoplasm of Radiolaria (q.v.), being differentiated into granular strands traversing a clear jelly, rich in large vacuoles (alveoli), and uniting outside the jelly to form the basal layer of the pseudopods; these again are radiolarian in character. Hence E.R. Lankester justly enough compares the shell here to the central capsule of the Radiolarian, though the comparison must not be pushed too far. The cytoplasm contains granules of various kinds, and the internal protoplasm is sometimes pigmented. The Chrysomonad Flagellate, Zooxanthella, so abundant in its resting state—the so-called “yellow cells”—in the extracapsular protoplasm of Radiolaria (q.v.) also occurs in the outer protoplasm of many Foraminifera, not only pelagic but also bottom-dwellers, such as Orbitolites.
The nucleus is single in the Nuda and Allogromidia and in the megalospheric forms of higher Foraminifera; but microspheric forms when adult contain many simple similar nuclei. The nucleus in every case gives off granules and irregular masses (“chromidia”) of similar reactions, which play an important part in reproduction. During the maturation of the microsphere the nuclei disappear; and the cytoplasm breaks up into a large number of zoospores, each of which is soon provided with a single nucleus, whether entirely derived from the parent-nucleus or from the coalescence of chromidia, or from both these sources is still uncertain. These zoospores are amoeboid; they soon secrete a shell and reveal themselves as megalospheres, the original state of the megalospheric forms. In the adult megalosphere the solitary nucleus disappears and is replaced by hosts of minute vesicular nuclei, formed by the concentration of chromidia. Each nucleus aggregates around it a proper zone of dense protoplasm; by two successive mitotic divisions each mass becomes quadri-nucleate, and splits up into four biflagellate, uninucleate zoospores. These are pairing-cells or gametes, though they will not pair with members of the same brood. In the zygote resulting from pairing two nuclei soon fuse into one; but this again divides into two; an embryonic shell is secreted, and this is the microspheric type, which is multinuclear from the first. F. Schaudinn compares the nuclei of the adult Foraminifera with the (vegetative) meganucleus of Infusora (q.v.) and the chromidial mass with the micronucleus, whose chief function is reproductive.
 |
| Fig. 14.—Vertical section of tubulated chamber-walls, a, a, of Nummulites. b, b, Marginal cord; c, cavity of chamber; d, d, non-tubulated columns. |
Since megalospheric forms are by far the most abundant, it seems probable that under most conditions they also give rise to megalospheric young like themselves; and that the production of zoospores, pairing to pass into the microspheric form, is only occasional, and possibly seasonal. This life-history we owe to the researches of Schaudinn and J.J. Lister.
In several species (notably Patellina) plastogamy, the union of the cytoplasmic bodies without nuclear fusion, has been noted, as a prelude to the resolution of the conjoined protoplasm into uninucleate amoebulae.
Calcituba, a porcellanous type, which after forming the embryonic chamber with its deflected pylome grows into branching stems, may fall apart into sections, or the protoplasm may escape and break up into small amoebulae. Of the reproduction of the simplest forms we know little. In Mikrogromia the cell undergoes fission within the test, and on its completion the daughter-cells may emerge as biflagellate zoospores.
The sandy shells are a very interesting series. In Astrorhiza the sand grains are loosely agglutinated, without mineral cement; they leave numerous pores for the exit of the protoplasm, and there are no true pylomes. In other forms the union of the grains by a calcareous or ferruginous cement necessitates the existence of distinct pylomes. Many of the species reproduce the varieties of form found in calcareous tests; some are finely perforated, others not. Many of the larger ones have their walls thickened internally and traversed by complex passages; this structure is called labyrinthic (fig. 19, g, h). The shell of Endothyra, a form only known to us by its abundance in Carboniferous and Triassic strata, is largely composed of calcite and is sometimes perforated.
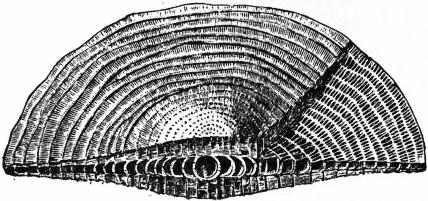 |
| Fig. 15.—Cycloclypeus. |
It is noteworthy that though of similar habitat each species selects its own size or sort of sand, some utilizing the siliceous spicules of sponges. Despite the roughness of the materials, they are often so laid as to yield a perfectly smooth inner wall; and sometimes the outer wall may be as simple. As we can find no record of a deflected stylopyle to the primitive chamber of the polythalamous Arenacea, it is safe to conclude that they have no close alliance with the Porcellanea.
Classification.
I. Nuda.—Protoplasmic body without any pellicle or shell save
in the resting encysted condition, sometimes forming
colonial aggregates by coalescence of pseudopods (Myxodictyum),
or even plasmodia (Protomyxa). Brood cells at
first uniflagellate or amoeboid from birth. Fresh-water
and marine genera Protogenes (Haeckel), Biomyxa (Leidy),
Myxodictyum (Haeckel), Protomyxa (Haeckel) (fig. 1B).
This group of very simple forms includes many of
Haeckel’s Monera, defined as “cytodes,” masses of protoplasm
without a nucleus. A nucleus (or nuclei) has,
however, been demonstrated by improved methods of
staining in so many that it is probable that this distinction
will fall to the ground.
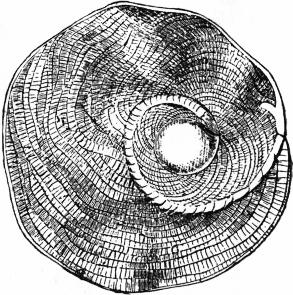 |
| Fig. 16.—Heterostegina. |
II. Allogromidiaceae (figs. 1A, 2).—Protoplasmic body protected in adult state by an imperforate test with one or two openings (pylomes) for the exit of the stylopod; test simple, gelatinous, membranous, sometimes incrusted with foreign bodies, never calcareous nor arenaceous; reproduction by fission alone known. Fresh-water or marine genera Allogromia (Rhumbl.), Myxotheca (Schaud.), Lieberkühnia (Cl. & L.) (fig. 1A), Shepheardella (Siddall) (fig. 2, 3-10), Diplophrys (Barker), Amphitrema (Arch.) (fig. 2, 11), Diaphorophodon (Arch.) (fig. 2, 12), are possibly Filosa. This group differs from the preceding in its simple test, but, like it, includes many fresh-water species, which possess contractile vacuoles.
III. Astrorhizidiaceae.—Simple forms, rarely polythalamous (some Rhabdamminidae), but often branching or radiate; test arenaceous, loosely compacted and traversed by chinks for pseudopodia (Astrorhizidae), or dense, and opening by one or more terminal pylomes at ends of branches. Marine, 4 Fam. The test of some Astrorhizidae is so loose that it falls to pieces when taken out of water. Haliphysema is remarkable for its history in relation to the “gastraea theory.” Pilulina has a neat globular shell of sponge-spicules and fine sand. Genera, Astrorhiza (Sandahl) (fig. 22), Pilulina (Carptr.) (fig. 19), Saccammina (Sars) (fig. 19), Rhabdammina (Sars), Botellina (Carptr.), Haliphysema (Bowerbank) (fig. 22).
IV. Lituolidaceae.—Shell arenaceous, usually fine-grained, definite and often polythalamic, recalling in structure calcareous forms. Lituola (Lamk.) (fig. 19), Endothyra (Phil.), Ammodiscus (Reuss), Loftusia (Brady), Haplophragmium (Reuss) (fig. 22), Thurammina (Brady) (fig. 22).
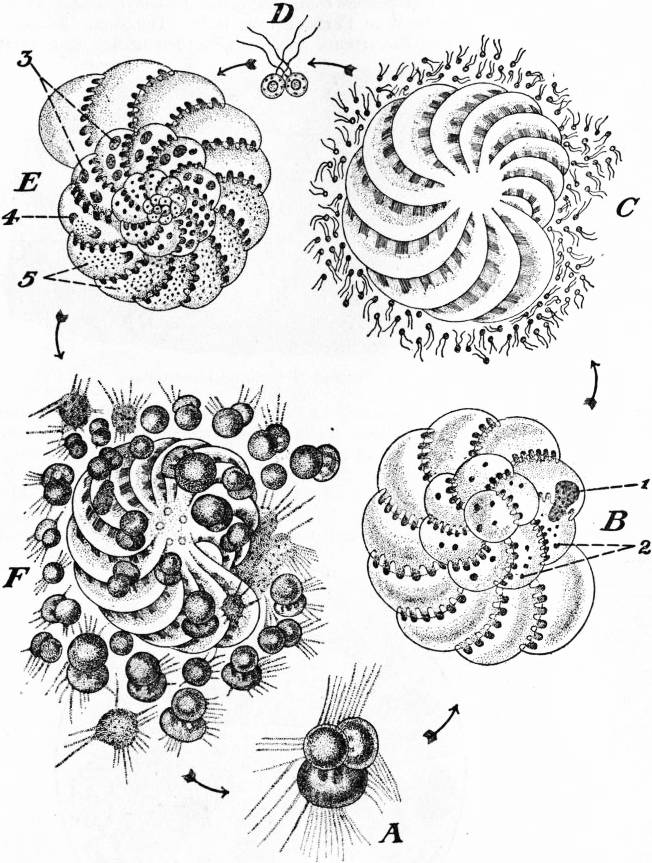 | |
| Modified from F. Schaudinn, in Lang’s Zoologie. | |
| Fig. 17.—Life Cycle of Polystomella crispa. | |
A, Young megalospheric individual. B, Adult decalcified. C, Later stage, resolving itself into two flagellate gametes. D, Conjugation. E, Microspheric individual produced from zygote. |
F, The same resolved itself into pseudopodiospores which are growing into new megalospheric individuals. 1, Principal nucleus, and 2, subsidiary nuclei of megalospheric form. 3, Nuclei. 4, Nuclei in multiple division. 5, Chromidia derived from 4. |
V. Miliolidaceae.—Shells porcellanous imperforate, almost invariably with a camptostyle leading from the embryonic chamber; Cornuspira (Schultze) (fig. 3); Miliola (Lamk.), including as subgenera Spiroloculina (d’Orb.) (figs. 3 and 22); Triloculina (d’Orb.) (fig. 3); Biloculina (d’Orb.) (fig. 3); Uniloculina (d’Orb.); Quinqueloculina (d’Orb.); Peneroplis (Montfort) (figs. 22, 3; 3), with form Dendritina (fig. 4, 1); Orbiculina (Lamk.) (fig. 3, 6-8); Orbitolites (Lamk.) (figs. 5, 6); Vertebralina (d’Orb.) (fig. 22); Squamulina (Sch.) (fig. 22); Calcituba (Schaudinn).
VI. Textulariadaceae.—Shells perforate, vitreous or (in the larger forms) arenaceous, in two or three alternating ranks (distichous or tristichous). Textularia (Defrance) (fig. 21).
VII. Cheilostomellaceae.—Shells vitreous, thin, the chambers doubling forwards and backwards as in Miliolidae. Cheilostomella (Reuss).
VIII. Lagenidaceae.—Shells vitreous, often sculptured, mono- or polythalamic, finely perforate; chambers flask-shaped, with a protruding or an inturned stylopyle; Lagena (Walker & Boys) (fig. 4, 9); Nodosaria (Lamk.) (figs. 23, 4; 4, 10); Polymorphina (d’Orb.) (fig. 4, 13); Cristellaria (Lamk.) (fig. 4, 11); Frondicularia (Def.) (fig. 23, 3).
IX. Globigerinidaceae.—Shells vitreous, coarsely perforated; chambers few spheroidal rapidly increasing in size; arranged in a trochoid or nautiloid spiral. Globigerina (Lamk.) (23, 6; 4, 12); Hastigerina (Wyville Thompson) (fig. 23, 5); Orbulina (d’Orb.) (fig. 23, 8).
X. Rotalidaceae.—Shells vitreous, finely perforate; walls thick, often double, but without an intermediate party-layer traversed by canals; form usually spiral or trochoid. Discorbina (Parker & Jones) (fig. 4, 15); Planorbulina (d’Orb.) (fig. 4, 17); Rotalia (Lamk.) (figs. 23, 1, 2; 7, 21); Calcarina (d’Orb.) (fig. 23, 10); Polytrema (Risso) (fig. 23, 9).
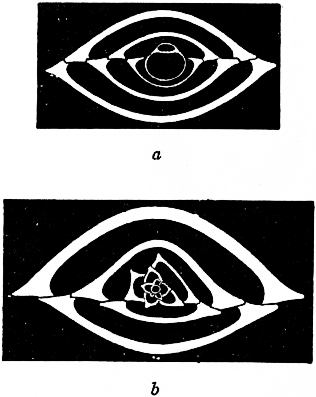 |
| Fig. 18.—Biloculina depressa d’Orb., transverse sections showing dimorphism. (From Lister.) |
a, Megalospheric shell × 50, showing uniform growth, biloculine throughout. b, Microspheric shell × 90, showing multiform growth, quinqueloculine at first, and then multiform. |
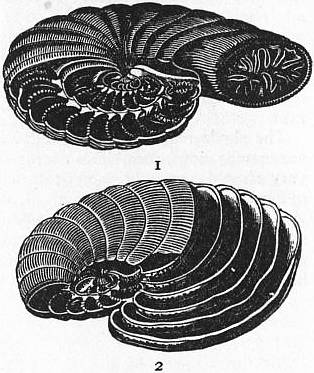
XI. Nummulinidaceae.—As in Rotalidaceae, but with a thicker finely perforated shell, often well developed, and a supplementary skeleton traversed by branching canals as an additional party-wall between the proper chamber-walls. Nonionina (d’Orb.) (fig. 4, 19); Fusulina (Fischer) (fig. 20); Polystomella (Lamk.) (figs. 4, 16; 8); Operculina (d’Orb.) (fig. 9); Heterostegina (d’Orb.) (fig. 16); Cycloclypeus (Carptr.) (fig. 15); Nummulites (Lamk.) (figs. 10, 11, 12, 13, 14).
“Eozoon canadense,” described as a species of this order by J.W. Dawson and Carpenter, has been pronounced by a series of enquirers, most of whom started with a belief in its organic structure, to be merely a complex mineral concretion in ophicalcite, a rock composed of an admixture of silicates (mostly serpentine and pyroxene) and calcite.
Distribution in Vertical Space.—Owing to their lack of organs for active locomotion the Foraminifera are all crawling or attached, with the exception of a few genera (very rich in species, however) which float near the surface of the ocean, constituting part of the pelagic plankton (q.v.). Thus the majority are littoral or deep-sea, sometimes attached to other bodies or even burrowing in the tests of other Foraminifera; most of the fresh-water forms are sapropelic, inhabiting the layer of organic débris at the surface of the bottom mud ditches of pools, ponds and lakes. The deep-sea species below a certain depth cannot possess a calcareous shell, for this would be dissolved; and it is in these that we find limesalts sometimes replaced by silica.
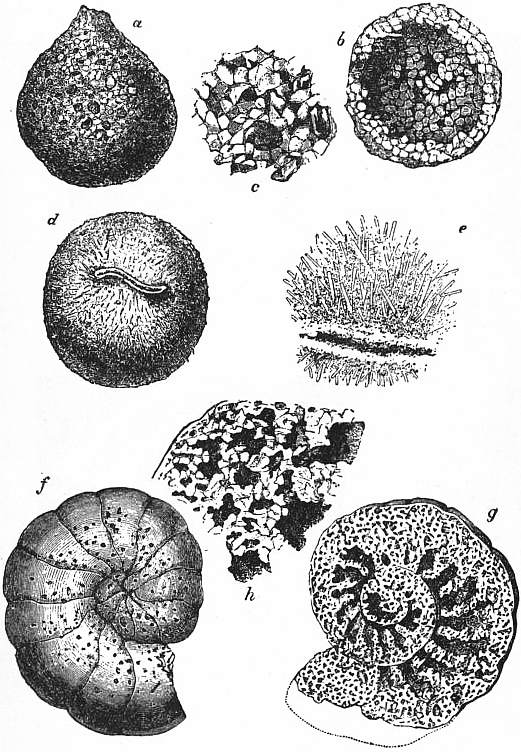 | |
| Fig. 19.—Arenaceous Foraminifera. | |
a, Exterior of Saccammina. b, The same laid open. c, Portion of test more highly magnified. d, Pilulina. |
e, Portion of test more highly magnified. f, Nautiloid Lituola, exterior. g, Chambered interior. h, Portion of labyrinthic chamber wall, showing component sand-grains. |
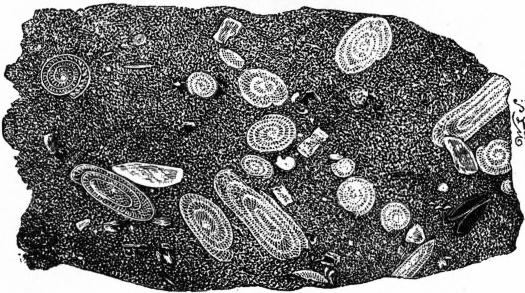 |
| Fig. 20.—Section of Fusulina Limestone. |
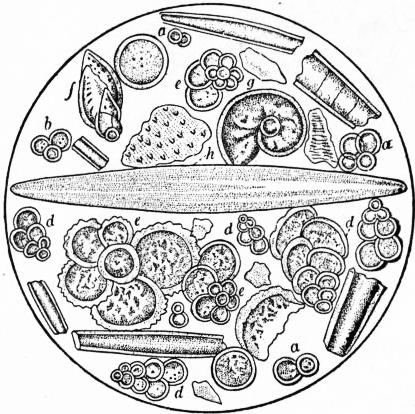 |
| Fig. 21.—Microscopic Organisms in Chalk from Gravesend. a, b, c, d, Textularia globulosa; e, e, e, e, Rotalia aspera; f, Textularia aculeata; g, Planularia hexas; h, Navicula. |
The pelagic floating genera are also specially modified. Their shell is either thin or extended many times by long slender tapering spines, and the protoplasm outside has the same character as that of the Radiolaria (q.v.), being differentiated into jelly containing enormous vacuoles and traversed by reticulate strands of granular protoplasm. These coalesce into a peripheral zone from which protrude the pseudopods, here rather radiate than reticulate. Most genera and most species are cosmopolitan; but local differences are often marked. Foraminifera abound in the shore sands and the crevices of coral reefs. The membranous shelled forms decay without leaving traces. The sandy or calcareous shells of dead Foraminifera constitute a large proportion of littoral sand, both below and above tide marks; and, as shown in the boring on Funafuti, enter largely into the constituents of coral rock. They may accumulate in the mud of the bottom to constitute Foraminiferal ooze. The source of these shells in the latter case is double: (1) shells of bottom-dwellers accumulate on the spot; (2) shells of dead plankton forms sink down in a continuous shower, to form a layer at the bottom of the ocean, during which process the spines are dissolved by the sea-water. Thus is formed an ooze known as “Globigerina-ooze,” being formed largely of that genus and its ally Hastigerina; below 3000 fathoms even the tests themselves are dissolved. Casts of their bodies in glauconite (a green ferrous silicate, whose composition has not yet been accurately determined) are, however, frequently left. Glauconitic casts of perforate shells, notably Globigerina, have been found in Lower Cambrian (e.g. Hollybush Sandstone), and the shells themselves in Siberian limestones of that age. It is only when we pass into the Silurian Wenlock limestone that sandy shells make their appearance. Above this horizon Foraminifera are more abundant as constituents, partial or principal of calcareous rocks, the genus Endothyra being indeed almost confined to Carboniferous beds. The genus Fusulina (fig. 20) and Saccammina (fig. 19) give their names (from their respective abundance) to two limestones of the Carboniferous series. Porcellanous shells become abundant only from the Lias upwards. The glauconitic grains of the Greensand formations are chiefly foraminiferal casts. Chalk is well known to consist largely of foraminiferal shells, mostly vitreous, like the north Atlantic globigerina ooze. In the Maestricht chalk more littoral conditions prevailed, and we find such large-sized species as Orbitoides (vitreous) and Orbitolites (porcellanous; figs. 5, 6), &c. In the Eocene Tertiaries the Calcaire Grossier of the Paris basin is mainly composed of Miliolid forms. Nummulites occur in English beds and in the Paris basin; but the great beds of these, forming reef-like masses of limestone, occur farther south, extending from the Pyrenees through the southern and eastern Alps to Egypt, Sinai, and on to north India. The peculiar structure occurring in the Lower Laurentian limestone, as well as other limestones of Archean age described as a Nummulitaceous genus, “Eozoon,” by Carpenter and Dawson, and abundantly illustrated in the 9th edition of his encyclopaedia, is now universally regarded as of inorganic origin. “Looking at the almost universal diffusion of existing Foraminifera and the continuous accumulation of their shells over vast areas of the ocean-bottom, they are certainly doing more than any other group of organisms to separate carbonate of lime from its solution in sea-water, so as to restore to the solid crust of the earth what is being continuously withdrawn from it by solution of the calcareous materials of the land above sea-level.” (E.R. Lankester, “Protozoa,” Ency. Brit. 9th ed.)
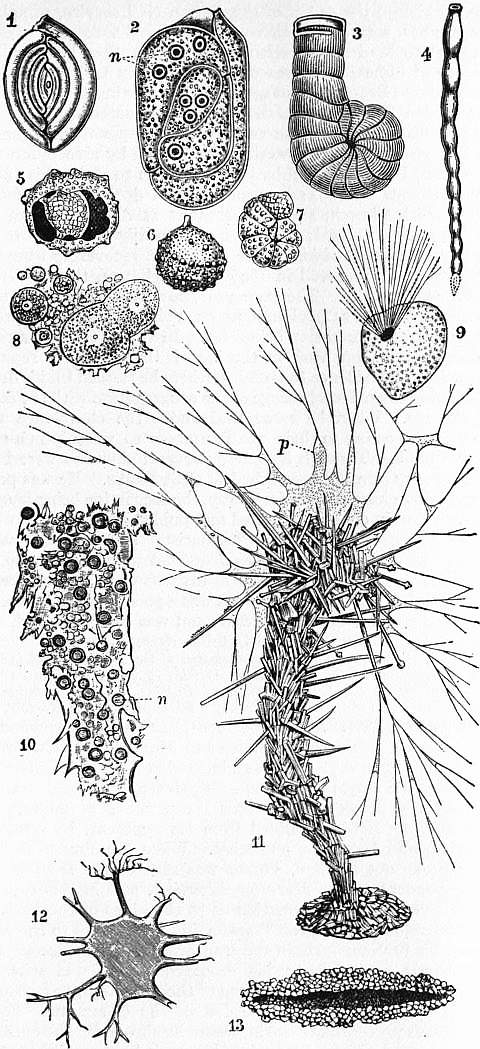 |
| Fig. 22.—Imperforata. |
|
1, Spiroloculina planulata, Lamarck, showing five “coils”; porcellanous. 2, Young ditto, with shell dissolved and protoplasm stained so as to show the seven nuclei n. 3, Spirolina (Peneroplis); a sculptured imperfectly coiled shell; porcellanous. 4, Vertebralina, a simple shell consisting of chambers succeeding one another in a straight line; porcellanous. 5, 6, Thurammina papillata, Brady, a sandy form. 5 is broken open so as to show an inner chamber; recent. × 25. 7, Haplophragmium canariensis, a sandy form; recent. 8, Nucleated reproductive bodies (bud-spores) of Haliphysema. 9, Squamulina laevis, M. Schultze; × 40; a simple porcellanous Miliolide. 10, Protoplasmic core removed after treatment with weak chromic acid from the shell of Haliphysema tumanovitzii, Bow. n, Vesicular nuclei, stained with haematoxylin. (After Lankester.) 11, Haliphysema tumanovitzii; × 25 diam.; living specimen, showing the wine-glass-shaped shell built up of sand-grains and sponge-spicules, and the abundant protoplasm p, issuing from the mouth of the shell and spreading partly over its projecting constituents. 12, Shell of Astrorhiza limicola, Sand.; × 3⁄2; showing the branching of the test on some of the rays usually broken away in preserved specimens (original). 13, Section of the shell of Marsipella, showing thick walls built of sand-grains. |
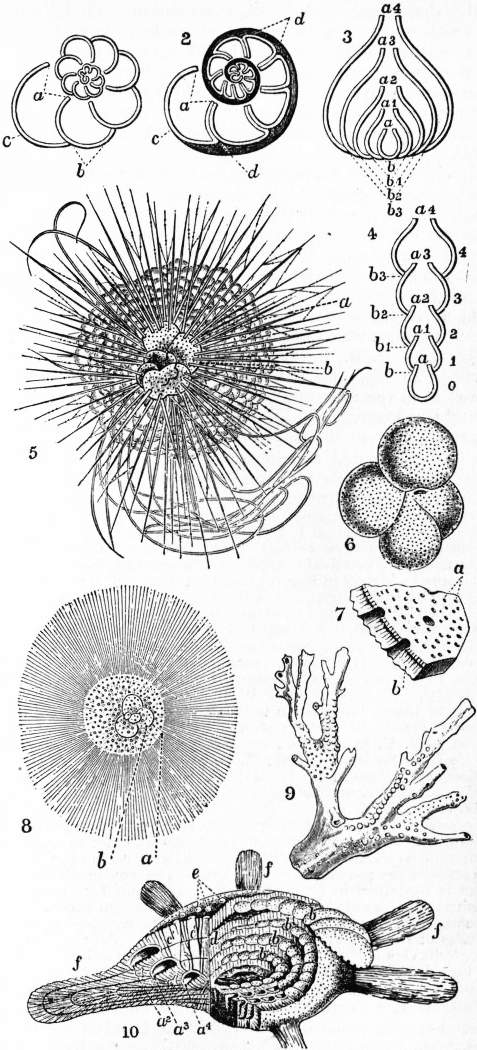 |
| Fig. 23.—Perforata. |
1, Spiral arrangement of simple chambers of a Reticularian shell, as in small Rotalia. 2, Ditto, with double septal walls, and supplemental shell-substance (shaded), as in large Rotalia. 3, Diagram to show the mode in which successively-formed chambers may completely embrace their predecessors, as in Frondicularia. 4, Diagram of a simple straight series of non-embracing chambers, as in Nodosaria. 5, Hastigerina murrayi, Wyv. Thomson, a, Bubbly (vacuolated) protoplasm, enclosing b, the perforated Globigerina-like shell (conf. central capsule of Radiolaria). From the peripheral protoplasm project, not only fine pseudopodia, but hollow spines of calcareous matter, which are set on the shell, and have an axis of active protoplasm. Pelagic; drawn in the living state. 6, Globigerina bulloides, d’Orb., showing the punctiform perforations of the shell and the main aperture. 7, Fragment of the shell of Globigerina, seen from within, and highly magnified, a, Fine perforations in the inner shell substances; b, outer (secondary) shell substance. Two coarser perforations are seen in section, and one lying among the smaller. 8, Orbulina universa, d’Orb. Pelagic example, with adherent radiating calcareous spines (hollow), and internally a small Globigerina shell. It is probably a developmental phase of Globigerina, a, Orbulina shell; b, Globigerina shell. 9, Polytrema miniaceum, Lin.; × 12. Mediterranean. Example of a branched adherent calcareous perforate Recticularian. 10, Calcarina spengleri, Gmel.; × 10. Tertiary, Sicily. Shell dissected so as to show the spiral arrangement of the chambers, and the copious secondary shell substance. a², a³, a4, Chambers of three successive coils in section, showing the thin primary wall (finely tubulate) of each; b, b, b, b, perforate surfaces of the primary wall of four tiers of chambers, from which the secondary shell substance has been cleared away; c′, c′, secondary or intermediate shell substance in section, showing coarse canals; d, section of secondary shell substance at right angles to c′; e, tubercles of secondary shell substance on the surface; f, f, club-like processes of secondary shell substance. |
Historical.—The Foraminifera were discovered as we have seen by A. d’Orbigny. C.E. Ehrenberg added a large number of species, but it was to F. Dujardin in 1835 that we owe the recognition of their true zoological position and the characters of the living animal. W.B. Carpenter and W.C. Williamson in England contributed largely to the study of the shell, the latter being the first to call attention to its multiform character in the development of a single species, and to utilize the method of thin sections, which has proved so fertile in results. W.K. Parker and H.B. Brady, separately, and in collaboration, described an enormous number of forms in a series of papers, as well as in the monograph by the latter of the Foraminifera of the “Challenger” expedition. Munier-Chalmas and Schlumberger brought out the fact of dimorphism in the group, which was later elucidated and incorporated in the full cytological study of the life-cycle of Foraminifera by J.J. Lister and F. Schaudinn, independently, but with concurrent results.
Literature.—The chief recent books are: F. Chapman, The Foraminifera (1902), and J.J. Lister, “The Foraminifera,” in E.R. Lankester’s Treatise on Zoology (1903), in which full bibliographies will be found. For a final résumé of the long controversy on Eozoon, see George P. Merrill in Report of the U.S. National Museum (1906), p. 635. Other classifications of the Foraminifera will be found by G.H. Theodor Eimer and C. Fickert in Zeitschr. für wissenschaftliche Zoologie, lxv. (1899), p. 599, and L. Rhumbler in Archiv für Protistenkunde, iii. (1903-1904); the account of the reproduction is based on the researches of J.J. Lister, summarized in the above-cited work, and of F. Schaudinn, in Arbeiten des kaiserlichen Gesundheitsamts, xix. (1903). We must also cite W.B. Carpenter, W.K. Parker and T. Rymer Jones, Introduction to the Study of the Foraminifera (Ray Society) (1862); W.B. Carpenter, “Foraminifera,” in Ency. Brit., 9th ed.; W.C. Williamson, On the Recent Foraminifera of Great Britain (Ray Society), (1858); H.B. Brady, “The Foraminifera,” in Challenger Reports, ix. (1884); A. Kemna, in Ann. de la soc. royale zoologique et malacologique de Belgique, xxxvii. (1902), p. 60; xxxix. (1904), p. 7.
Appendix.—The Xenophyophoridae are a small group of bottom-dwelling Sarcodina which show a certain resemblance to arenaceous Foraminifera, though observations in the living state show that the character of the pseudopodia is lacking. The multinucleate protoplasm is contained in branching tubes, aggregated into masses of definite form, bounded by a common wall of foreign bodies (sponge spicules, &c.) cemented into a membrane. The cytoplasm contains granules of BaSO4 and pellets of faecal matter. All that is known of reproduction is the resolution of the pellets into uninucleate cells. (F.E. Schultze, Wissenschaftliche Ergebnisse der deutschen Tiefsee-Expedition, vol. xi., 1905, pt. i.)
↧ Download as ZWI file | Last modified: 11/17/2022 15:24:15 | 7 views
☰ Source: https://oldpedia.org/article/britannica11/Foraminifera | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF