Dids
 From Handwiki
From Handwiki 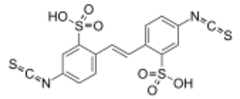
| |
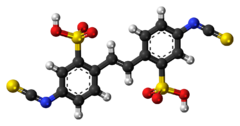
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2′-(Ethene-1,2-diyl)bis(5-isothiocyanatobenzene-1-sulfonic acid) | |
| Other names
5-Isothiocyanato-2-[2-(4-isothiocyanato-2-sulfophenyl)ethenyl]benzenesulfonic acid
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| Abbreviations | DIDS |
Beilstein Reference
|
6543839 |
| ChEBI |
|
| ChemSpider |
|
IUPHAR/BPS
|
|
| KEGG |
|
| MeSH | 4,4'-Diisothiocyanostilbene-2,2'-disulfonic+acid |
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C16H10N2O6S4 |
| Molar mass | 454.50 g·mol−1 |
| Melting point | 400 °C (752 °F; 673 K) |
| log P | 4.72 |
| Acidity (pKa) | -3.21, -1.428, -0.37, 0.23 |
| Basicity (pKb) | 13.77, 14.37, 15.425, 17.21 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
4,4′-Diisothiocyano-2,2′-stilbenedisulfonic acid (DIDS) is an anion exchange inhibitor,[1] blocking reversibly, and later irreversibly, exchangers such as chloride-bicarbonate exchanger.[2]
References
- ↑ Jessen, Flemming; Sjøholm, C; Hoffmann, EK (1986), "Identification of the anion exchange protein of ehrlich cells: A kinetic analysis of the inhibitory effects of 4,4′-diisothiocyano-2,2′-stilbene-disulfonic acid (DIDS) and labeling of membrane proteins with3H-DIDS", Journal of Membrane Biology 92 (3): 195–205, doi:10.1007/BF01869388, PMID 3783658
- ↑ Lane, Michelle; Baltz, Jay M.; Bavister, Barry D. (1999), "Bicarbonate/Chloride Exchange Regulates Intracellular pH of Embryos but Not Oocytes of the Hamster", Biology of Reproduction 61 (2): 452–457, doi:10.1095/biolreprod61.2.452, PMID 10411526
 |
Categories: [Sulfonic acids] [Isothiocyanates] [Alkene derivatives]
↧ Download as ZWI file | Last modified: 11/06/2023 05:56:16 | 3 views
☰ Source: https://handwiki.org/wiki/Chemistry:DIDS | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF