Immune System
 From Nwe
From Nwe The immune system is the system of specialized cells and organs that protects an organism from outside biological influences (though in a broad sense, almost every organ has a protective function—for example, the tight seal of the skin or the acidic environment of the stomach).
When the immune system is functioning properly, it protects the body against bacteria and viral infections and destroys cancer cells and foreign substances. If the immune system weakens, its ability to defend the body also weakens, allowing pathogens (infectious agents), including viruses that cause common colds and flu, to survive and flourish in the body. Because the immune system also performs surveillance of tumor cells, immune suppression has been reported to increase the risk of certain types of cancer.
The complex coordination of the immune system is stunning. It is capable of recognizing millions of invaders and neutralizing their attacks, and yet at the same time it allows helpful, symbiotic bacteria, such as E. coli, to become established within the human body. From the time of the initial invasion of a foreign element until its removal, the entire immune system—including diverse types of white blood cells, each with a different responsibility—harmoniously functions together in recognizing, attacking, and destroying substances identified as foreign.
The immune system is often divided into two sections:
- Innate immunity: Comprised of hereditary (always there) components that provide an immediate "first-line" of defense to continuously ward off pathogens.
- Adaptive (acquired) immunity: By manufacturing a class of proteins called antibodies, and by producing T-cells specifically designed to target particular pathogens, the body can develop a specific immunity to particular pathogens. This response takes days to develop, and so is not effective at preventing an initial invasion, but it will normally prevent any subsequent infection, and also aids in clearing up longer-lasting infections.
Another way of categorizing this is "nonspecific defenses" (skin, mucous membranes, phagocytes, fever, interferons, cilia, and stomach acid) and "specific defenses" (the cell-mediated and the humoral systems, both of which attack specific pathogens).
Adaptive immune system
The adaptive immune system, also called the "acquired immune system, and "specific immune system," ensures that animals that survive an initial infection by a pathogen are generally immune to further illness caused by that same pathogen. The adaptive immune system is based on dedicated immune cells termed leukocytes (white blood cells).
The basis of specific immunity lies in the capacity of immune cells to distinguish between proteins produced by the body's own cells ("self" antigen—those of the original organism), and proteins produced by invaders or cells under control of a virus ("non-self" antigen—or, what is not recognized as the original organism). This distinction is made via T-Cell Receptors (TCR) or B-Cell Receptors (BCR). For these receptors to be efficient they must be produced in thousands of configurations; this way they are able to distinguish between many different invader proteins.
This immense diversity of receptors would not fit in the genome of a cell, and millions of genes, one for each type of possible receptor, would be impractical. Instead, there are a few families of genes, each one having a slightly different modification. Through a special process, unique to cells of jawed vertebrates (Gnathostomata), the genes in these T-cell and B-cell lymphocytes recombine, one from each family, arbitrarily into a single gene.
This way, for example, each antibody or BCR of B lymphocytes has six portions, and is created from two genes unique to this lymphocyte, created by the recombination (union) of a random gene from each family. If there are 6 families, with 50, 30, 9, 40, and 5 members, the total possible number of antibodies is 50x30x6x9x40x5 = 16 million. On top of this there are other complex processes that increase the diversity of BCR or TCR even more, by mutation of the genes in question. The variability of antibodies is practically limitless, and the immune system creates antibodies for any molecule, even artificial molecules that do not exist in nature.
Many TCR and BCR created this way will react with their own peptides. One of the functions of the thymus and bone marrow is to hold young lymphocytes until it is possible to determine which ones react to molecules of the organism itself. This is done by specialized cells in these organs that present the young lymphocytes with molecules produced by them (and effectively the body). All the lymphocytes that react to them are destroyed, and only those that show themselves to be indifferent to the body are released into the bloodstream.
The lymphocytes that do not react to the body number in the millions, each with millions of possible configurations of receptors, each with a receptor for different parts of each microbial protein possible. The vast majority of lymphocytes never find a protein that its receptor is specified for, those few that do find one are stimulated to reproduce. Effective cells are generated with the specific receptor and memory cells. These memory cells are quiescent, they have long lives and are capable of identifying this antigen some time later, multiplying themselves quickly and rapidly responding to future infections.
In many species, the adaptive immune system can be divided into two major sections, the humoral immune system and the cell-mediated immune system.
Humoral immune system
The humoral immune system acts against bacteria and viruses in the body liquids (e.g., blood) by means of proteins, called immunoglobulins (also known as antibodies), which are produced by B cells. B cells are lymphocytes, with the "B" standing for the bursa of Fabricius, an organ unique to birds, where avian B cells mature. (It does not stand for bone marrow, where B cells are produced in all other vertebrates except for rabbits. B cells were original observed in studies done on immunity in chickens.)
Secreted antibodies bind to antigens on the surfaces of invading microbes (such as viruses or bacteria), which flags them for destruction. An antigen is any substance that causes the immune system to produce antibodies.
Humoral immunity refers to antibody production and all the accessory processes that accompany it: Th2 (T-helper 2 cells) activation and cytokine production (cytokines are proteins that affect the interaction between cells); germinal center formation and isotype switching (switching a specific region of the antibody); and affinity maturation and memory cell generation (memory cell generation has to do with the ability for a body to "remember" a pathogen by producing antibodies specifically targeted for it). Humoral immunity also refers to the effector functions of antibodies, which include pathogen and toxin neutralization, classical complement activation, and opsonin promotion of phagocytosis and pathogen elimination.
The human body has the ability to form millions of different types of B cells each day, and each type has a unique receptor protein, referred to as the B cell receptor (BCR), on its membrane that will bind to one particular antigen. At any one time in the human body there are B cells circulating in the blood and lymph, but are not producing antibodies. Once a B cell encounters its cognate antigen and receives an additional signal from a helper T cell, it can further differentiate into one of two types of B cells.
B cells need two signals to initiate activation. Most antigens are T-dependent, meaning T cell help is required for maximum antibody production. With a T-dependent antigen, the first signal comes from antigen cross linking BCR (B cell receptor) and the second from the Th2 cell. T-dependent antigens present peptides on B cell Class II MHC proteins to Th2 cells. This triggers B cell proliferation and differentiation into plasma cells. Isotype switching to IgG, IgA, and IgE and memory cell generation occur in response to T-dependent antigens.
Some antigens are T-independent, meaning they can deliver both the antigen and the second signal to the B cell. Mice without a thymus (nude or athymic mice) can respond to T-independent antigens. Many bacteria have repeating carbohydrate epitopes that stimulate B cells to respond with IgM synthesis in the absence of T cell help.
T-dependent responses require that B cells and their Th2 cells respond to epitopes on the same antigen. T and B cell epitopes are not necessarily identical. (Once virus-infected cells have been killed and unassembled virus proteins released, B cells specific for internal proteins can also be activated to make opsonizing antibodies to those proteins.) Attaching a carbohydrate to a protein can convert the carbohydrate into a T-dependent antigen; the carbohydrate-specific B cell internalizes the complex and presents peptides to Th2 cells, which in turn activate the B cell to make antibodies specific for the carbohydrate.
Antibodies
An antibody is a large Y-shaped protein used to identify and neutralize foreign objects like bacteria and viruses. Production of antibodies and associated processes constitutes the humoral immune system. Each antibody recognizes a specific antigen unique to its target. This is because at the two tips of its "Y," it has structures akin to locks. Every lock only has one key, in this case, its own antigen. When the key is inserted into the lock, the antibody activates, tagging or neutralizing its target. The production of antibodies is the main function of the humoral immune system.
Immunoglobulins are glycoproteins in the immunoglobulin superfamily that function as antibodies. The terms antibody and immunoglobulin are often used interchangeably. They are found in the blood and tissue fluids, as well as many secretions. In structure, they are globulins (in the γ-region of protein electrophoresis). They are synthesized and secreted by plasma cells that are derived from the B cells of the immune system. B cells are activated upon binding to their specific antigen and differentiate into plasma cells. In some cases, the interaction of the B cell with a T helper cell is also necessary.
In humans, there are five types: IgA, IgD, IgE, IgG, and IgM. (Ig stands for immunoglobulin.). This is according to differences in their heavy chain constant domains. (The isotypes are also defined with light chains, but they do not define classes, so they are often neglected.) Other immune cells partner with antibodies to eliminate pathogens depending on which IgG, IgA, IgM, IgD, and IgE constant binding domain receptors it can express on its surface.
The antibodies that a single B lymphocyte produces can differ in their heavy chain, and the B cell often expresses different classes of antibodies at the same time. However, they are identical in their specificity for antigen, conferred by their variable region. To achieve the large number of specificities the body needs to protect itself against many different foreign antigens, it must produce millions of B lymphoyctes. In order to produce such a diversity of antigen binding sites for each possible antigen, the immune system would require many more genes than exist in the genome. It was Susumu Tonegawa who showed in 1976 that portions of the genome in B lymphocytes can recombine to form all the variation seen in the antibodies and more. Tonegawa won the Nobel Prize in Physiology or Medicine in 1987 for his discovery.
Cell-mediated immune system
The cell-mediated immune system, the second main mechanism of the adaptive immune system, destroys virus-infected cells (among other duties) with T cells, also called "T lymphocytes." ("T" stands for thymus, where their final stage of development occurs.)
Cell-mediated immunity is an immune response that does not involve antibodies but rather involves the activation of macrophages and natural killer cells, the production of antigen-specific cytotoxic T-lymphocytes, and the release of various cytokines in response to an antigen. Cellular immunity protects the body by:
- activating antigen-specific cytotoxic T-lymphocytes that are able to lyse body cells displaying epitopes (sections of protein) of foreign antigen on their surface, such as virus-infected cells, cells with intracellular bacteria, and cancer cells displaying tumor antigens;
- activating macrophages and natural killer cells, enabling them to destroy intracellular pathogens; and
- stimulating cells to secrete a variety of cytokines that influence the function of other cells involved in adaptive immune responses and innate immune responses.
Cell-mediated immunity is directed primarily at microbes that survive in phagocytes and microbes that infect non-phagocytic cells. It is most effective in removing virus-infected cells, but also participates in defending against fungi, protozoans, cancers, and intracellular bacteria. It also plays a major role in transplant rejection.
There are two major types of T cells:
- Cytotoxic T cells (CD8 cells). A cytotoxic T cell (also known as TC or killer T cell) is a sub-group of T lymphocyte (a type of white blood cell) which is capable of inducing the death of infected somatic or tumor cells; they kill cells that are infected with viruses (or other pathogens), or are otherwise damaged or dysfunctional. These cells recognize infected cells by using T cell receptors to probe cell surfaces. If they recognize an infected cell, they release granzymes to trigger that cell to become apoptotic ("commit suicide"), thus killing that cell and any viruses that it is in the process of creating; they also release perforins, which perforate the infected cell's membrane, exposing its contents to the often hostile extracellular environment.
- Helper T cells (CD4 cells). CD4+ Lymphocytes, or helper T cells, are immune response controllers. They "decide" which actions to take during an invasion, promoting or inhibiting all other immune cells via cytokines. These cells activate macrophages (cells that ingest dangerous material), and also produce cytokines (interleukins) that induce the proliferation of B and T cells. HIV, being a virus that directly attacks the CD4+ T cells, causes a collapse of the entire system by attacking the root.
In addition, there are regulatory T cells (Treg cells) which are important in regulating cell-mediated immunity.
Innate immune system
The adaptive immune system could take days or weeks after an initial infection to have an effect. However, most organisms are under constant assault from pathogens that must be kept in check by the faster-acting innate immune system. Innate immunity, or non-specific defense, defends against pathogens by rapid responses coordinated through chemical or physical barriers or "innate" receptors that recognize a wide spectrum of conserved pathogenic components.
In evolutionary time, it appears that the adaptive immune system developed abruptly in jawed fish. Prior to jawed fish, there is no evidence of adaptive immunity, and animals therefore relied only on their innate immunity. Plants, on the other hand, rely on secondary metabolites (chemical compounds in organisms that are not directly involved in the normal growth, development, or reproduction of organisms) to defend themselves against fungal and viral pathogens as well as insect herbivory. Plant secondary metabolites are derived through vast arrays of plant biosynthetic pathways not needed directly for plant survival, hence why they are named secondary. Plant secondary metabolism should not be confused with innate or adaptive immunity as they evolved along an entirely different evolutionary lineages and rely on entirely different signal cues, pathways, and responses.
The innate immune system, when activated, has a wide array of effector cells and mechanisms. There are several different types of phagocytic cells, which ingest and destroy invading pathogens. The most common phagocytes are neutrophils, macrophages, and dendritic cells. Another cell type, natural killer cells, are especially adept at destroying cells infected with viruses. Another component of the innate immune system is known as the complement system. Complement proteins are normally inactive components of the blood. However, when activated by the recognition of a pathogen or antibody, the various proteins recruit inflammatory cells, coat pathogens to make them more easily phagocytosed, and make destructive pores in the surfaces of pathogens.
First-line defense: physical and chemical barrier
The first-line defense includes barriers to infection, such as skin, the mucous coating of the gut, and airways. These physically prevent the interaction between the host and the pathogen. Pathogens that penetrate these barriers encounter constitutively expressed (constantly expressed) anti-microbial molecules (e.g., lysozymes) that restrict the infection.
In addition to the usual defense, the stomach secretes gastric acid, which, in addition to aiding digestive enzymes in the stomach to work on food, prevents bacterial colonization by most pathogens.
Second-line defense: Phagocytic cells
The second-line defense includes phagocytic cells (macrophages and neutrophil granulocytes) that can engulf (phagocytose) foreign substances. Macrophages are thought to mature continuously from circulating monocytes.
Phagocytosis involves chemotaxis, where phagocytic cells are attracted to microorganisms by means of chemotactic chemicals such as microbial products, complement, damaged cells, and white blood cell fragments. Chemotaxis is followed by adhesion, where the phagocyte sticks to the microorganism. Adhesion is enhanced by opsonization, where proteins like opsonins are coated on the surface of the bacterium. This is followed by ingestion, in which the phagocyte extends projections, forming pseudopods that engulf the foreign organism. Finally, the bacterium is digested by the enzymes in the lysosome, exposing it to reactive oxygen species and proteases.
Anti-microbial proteins
In addition, anti-microbial proteins may be activated if a pathogen passes through the barrier offered by skin. There are several classes of antimicrobial proteins, such as acute phase proteins (C-reactive protein, for example, enhances phagocytosis and activates complement when it binds itself to the C-protein of S. pneumoniae ), lysozyme, and the complement system.
The complement system is a very complex group of serum proteins, which is activated in a cascade fashion. Three different pathways are involved in complement activation:
- classical pathway: recognizes antigen-antibody complexes
- alternative pathway: spontaneously activates on contact with pathogenic cell surfaces
- mannose-binding lectin pathway: recognizes mannose sugars, which tend to appear only on pathogenic cell surfaces.
A cascade of protein activity follows complement activation; this cascade can result in a variety of effects, including opsonization of the pathogen, destruction of the pathogen by the formation and activation of the membrane attack complex, and inflammation.
Interferons are also anti-microbial proteins. These molecules are proteins that are secreted by virus-infected cells. These proteins then diffuse rapidly to neighboring cells, inducing the cells to inhibit the spread of the viral infection. Essentially, these anti-microbial proteins act to prevent the cell-to-cell proliferation of viruses.
Research
Earlier studies of innate immunity utilized model organisms that lack adaptive immunity, such as the plant Arabidopsis thaliana, the fly Drosophila melanogaster, and the worm Caenorhabditis elegans. Advances have since been made in the field of innate immunology with the discovery of toll-like receptors (TLRs) and the intracellular nucleotide-binding site leucine-rich repeat proteins (NODs). NODs are receptors in mammal cells that are responsible for a large proportion of the innate immune recognition of pathogens.
In 1989, prior to the discovery of mammalian TLRs, Charles Janeway conceptualized and proposed that evolutionarily conserved features of infectious organisms were detected by the immune system through a set of specialized receptors, which he termed pathogen-associated molecular patterns (PAMPs) and pattern recognition receptors (PRRs), respectively. This insight was only fully appreciated after the discovery of TLRs by the Janeway lab in 1997. The TLRs now comprise the largest family of innate immune receptors (or PRRs). Janeway’s hypothesis has come to be known as the "stranger model" and substantial debate in the field persists to this day as to whether or not the concept of PAMPs and PRRs, as described by Janeway, is truly suitable to describe the mechanisms of innate immunity. The competing "danger model" was proposed in 1994 by Polly Matzinger and argues against the focus of the stranger model on microbial derived signals, suggesting instead that endogenous danger/alarm signals from distressed tissues serve as the principle purveyors of innate immune responses.
Both models are supported in the later literature, with discoveries that substances of both microbial and non-microbial sources are able to stimulate innate immune responses, which has led to increasing awareness that perhaps a blend of the two models would best serve to describe the currently known mechanisms governing innate immunity.
Intersections between systems
Splitting the immune system into innate and adaptive systems simplifies discussions of immunology. However, the systems actually are quite intertwined in a number of important respects.
One important example is the mechanisms of "antigen presentation." After they leave the thymus, T cells require activation to proliferate and differentiate into cytotoxic ("killer") T cells (CTLs). Activation is provided by antigen-presenting cells (APCs), a major category of which are the dendritic cells. These cells are part of the innate immune system.
Activation occurs when a dendritic cell simultaneously binds itself to a T "helper" cell's antigen receptor and to its CD28 receptor, which provides the "second signal" needed for DC activation. This signal is a means by which the dendritic cell conveys that the antigen is indeed dangerous, and that the next encountered T "killer" cells need to be activated. This mechanism is based on antigen-danger evaluation by the T cells that belong to the adaptive immune system. But the dendritic cells are often directly activated by engaging their toll-like receptors, getting their "second signal" directly from the antigen. In this way, they actually recognize in "first person" the danger, and direct the T killer attack. In this respect, the innate immune system therefore plays a critical role in the activation of the adaptive immune system.
Adjuvants, or chemicals that stimulate an immune response, provide artificially this "second signal" in procedures when an antigen that would not normally raise an immune response is artificially introduced into a host. With the adjuvant, the response is much more robust. Historically, a commonly-used formula is Freund's Complete Adjuvant, an emulsion of oil and mycobacterium. It was later discovered that toll-like receptors, expressed on innate immune cells, are critical in the activation of adaptive immunity.
Other factors that affect immune response
Many factors can contribute to the general weakening of the immune system:
- Malnutrition (unbalanced diet/poor eating habits that cause a lack of vitamins and minerals)
- Alcohol abuse
- Drug abuse, either intravenous or other (appears related to associated factors i.e. poor diet, use of infected/dirty needles, poor exercise, stress/depression)
- Medications (particularly the use of anti-cancer drugs, corticosteroids, and antibiotics)
- Radiation
- Exposure to certain environmental toxins, whether naturally occurring or from pollution. These include:
- Cigarette smoke
- Stress/Depression - Research shows that psychological stress can greatly increase your susceptibility to colds and other viral diseases, namely through an increase in serum corticosteroid levels
- Age - Ability of the immune system to respond is decreased at early and old age.
- Disease or medications (i.e. Diabetes, corticosteroids, immune suppressant drugs), causing constant exposure to infectious agents without natural defense (intact skin)
- Inadequate sleep at the Delta brain wave level.
- Lack of exercise as well as excessive exercise resulting in physiological stress
- Long-term weightlessness
- Diseases either infectious or other causing more depression on the immune system like:
- Cancer, and hematological malignancy (such as leukemia, lymphoma and myeloma) in particular.
- Diabetes Mellitus
- Cystic fibrosis
- Lupus Erythematosus
- Nephrotic syndrome
- Viral infections i.e. viral respiratory infections then allowing for bacterial pneumonia to develop.
- HIV
- Ulcerative colitis
- Bulimia (due to malnutrition, stress, depression).
- Sickle-cell disease.
- Liver disease/cirrhosis
- Cushing's syndrome
Pharmacology
Despite high hopes, there are no medications that directly increase the activity of the immune system. Various forms of medication that activate the immune system may cause autoimmune disorders.
Suppression of the immune system is often used to control autoimmune disorders or inflammation when this causes excessive tissue damage, and to prevent transplant rejection after an organ transplant. Commonly used immunosuppressants include glucocorticoids, azathioprine, methotrexate, ciclosporin, cyclophosphamide, and mercaptopurine. In organ transplants, ciclosporin, tacrolimus, mycophenolate mofetil, and various others are used to prevent organ rejection through selective T cell inhibition.
Syndromes of the human immune system
The most important function of the human immune system occurs at the cellular level of the blood and tissues. The lymphatic and blood circulation systems are highways for specialized white blood cells to travel around the body. Each white blood cell type (B cells, T cells, natural killer cells, and macrophages) has a different responsibility, but all function together with the primary objective of recognizing, attacking, and destroying bacteria, viruses, cancer cells, and all substances seen as foreign. Without this coordinated effort, a person would not be able to survive more than a few days before succumbing to overwhelming infection.
Infections set off an alarm that alerts the immune system to bring out its defensive weapons. Natural killer cells and macrophages rush to the scene to consume and digest infected cells. If the first line of defense fails to control the threat, antibodies, produced by the B cells, upon the order of T helper cells, are custom-designed to hone in on the invader.
Many disorders of the human immune system fall into two broad categories that are characterized by:
- Attenuated immune response. There are "congenital" (inborn) and "acquired" forms of immunodeficiency, characterized by an attenuated response. Chronic granulomatous disease, in which phagocytes have trouble destroying pathogens, is an example of the former, while AIDS ("Acquired Immune Deficiency Syndrome"), an infectious disease tied to the HIV virus that destroys CD4+ T cells, is an example of the latter. Immunosuppressive medication intentionally induces an immunodeficiency in order to prevent rejection of transplanted organs.
- Overzealous immune response. On the other end of the scale, an overactive immune system figures in a number of other disorders, particularly autoimmune disorders such as lupus erythematosus, type I diabetes (sometimes called "juvenile onset diabetes"), multiple sclerosis, psoriasis, and rheumatoid arthritis. In these, the immune system fails to properly distinguish between self and non-self, and attacks a part of the patient's own body. Other examples of overzealous immune responses in disease include hypersensitivities, such as allergies and asthma.
References
ISBN links support NWE through referral fees
- Coico, R., G. Sunshine, and E. Benjamini. 2003. Immunology: A Short Course 5th Edition. Wiley-Liss. ISBN 04771226890
- Janeway, C., and P. Travers. 2004. Immunobiology. (Sixth Edition). Garland-Current Biology. ISBN 0815341016
- Lichtman, A. 2006. The Immunology. Retrieved May 25, 2007.
- Roitt, I., and P. J. Delves. 2001. Essential Immunology. Blackwell ISBN 0-632-05902-8
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Immune_system history
- Cell-mediated_immunity history
- Humoral_immunity history
- Secondary_metabolite history
- B_cell history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 11:57:27 | 83 views
☰ Source: https://www.newworldencyclopedia.org/entry/Cell-mediated_immunity | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: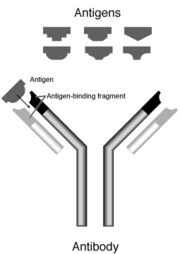
 KSF
KSF