Terbium(Iii) Oxide
 From Handwiki
From Handwiki 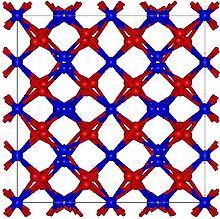
| |
| Names | |
|---|---|
| IUPAC name
terbium(III) oxide
| |
| Other names
terbium trioxide, terbia, terbium sesquioxide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
O3Tb2 |
| Molar mass | 365.848 g·mol−1 |
| Appearance | white crystals |
| Density | 7.91 g/cm3 |
| Melting point | 2,410 °C (4,370 °F; 2,680 K) |
Magnetic susceptibility (χ)
|
0.07834 cm3/mol |
| Structure | |
Crystal structure
|
Cubic, cI80 |
Space group
|
Ia3, No. 206[1] |
Lattice constant
|
a = 1.057 nm
|
| Thermochemistry | |
Std molar
entropy (S |
156.90 J/mol·K [2] |
Std enthalpy of
formation (ΔfH⦵298) |
-1865.23 kJ/mol [2] |
Gibbs free energy (ΔfG˚)
|
-1776.553 kJ/mol [2] |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
GHS hazard statements
|
H319, H400, H410 |
GHS precautionary statements
|
P264, P273, P280, P305+351+338, P337+313, P391, P501 |
| Related compounds | |
Other anions
|
Terbium(III) chloride |
Other cations
|
Gadolinium(III) oxide Dysprosium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Terbium(III) oxide, also known as terbium sesquioxide, is a sesquioxide of the rare earth metal terbium, having chemical formula Tb2O3. It is a p-type semiconductor, which conducts protons, which is enhanced when doped with calcium.[3] It may be prepared by the reduction of Tb4O7 in hydrogen at 1300 °C for 24 hours.[4]
- Tb
4O
7 + H
2 → 2 Tb
2O
3 + H
2O
It is a basic oxide and easily dissolved to dilute acids, and then almost colourless terbium salt is formed.
- Tb2O3 + 6 H+ → 2 Tb3+ + 3 H2O
The crystal structure is cubic and the lattice constant is a = 1057 pm.[5]
References
- ↑ Curzon A.E.; Chlebek H.G. (1973). "The observation of face centred cubic Gd, Tb, Dy, Ho, Er and Tm in the form of thin films and their oxidation". J. Phys. F 3 (1): 1–5. doi:10.1088/0305-4608/3/1/009.
- ↑ 2.0 2.1 2.2 R. Robie, B. Hemingway, and J. Fisher, "Thermodynamic Properties of Minerals and Related Substances at 298.15K and 1bar Pressure and at Higher Temperatures," US Geol. Surv., vol. 1452, 1978.[1]
- ↑ Reidar Haugsrud; Yngve Larring; Truls Norby (December 2005). "Proton conductivity of Ca-doped Tb2O3". Solid State Ionics (Elsevier B.V.) 176 (39–40): 2957–2961. doi:10.1016/j.ssi.2005.09.030.Tb2O3&rft.jtitle=Solid+State+Ionics&rft.aulast=Reidar+Haugsrud&rft.au=Reidar+Haugsrud&rft.au=Yngve+Larring&rft.au=Truls+Norby&rft.date=December+2005&rft.volume=176&rft.issue=39–40&rft.pages=2957–2961&rft.pub=Elsevier+B.V.&rft_id=info:doi/10.1016/j.ssi.2005.09.030&rfr_id=info:sid/en.wikibooks.org:Chemistry:Terbium(III)_oxide">
- ↑ G. J. McCarthy (October 1971). "Crystal data on C-type terbium sesquioxide (Tb2O3)". Journal of Applied Crystallography 4 (5): 399–400. doi:10.1107/S0021889871007295.Tb2O3)&rft.jtitle=Journal+of+Applied+Crystallography&rft.aulast=G.+J.+McCarthy&rft.au=G.+J.+McCarthy&rft.date=October+1971&rft.volume=4&rft.issue=5&rft.pages=399–400&rft_id=info:doi/10.1107/S0021889871007295&rfr_id=info:sid/en.wikibooks.org:Chemistry:Terbium(III)_oxide">
- ↑ N. C. Baenzinger, H. A. Eick, H. S. Schuldt, L. Eyring: Terbium Oxides. III. X-Ray Diffraction Studies of Several Stable Phases. In: Journal of the American Chemical Society, 1961, 83, 10, S. 2219-23.
Categories: [Terbium compounds] [Semiconductor materials]
↧ Download as ZWI file | Last modified: 09/28/2022 15:30:27 | 3 views
☰ Source: https://handwiki.org/wiki/Chemistry:Terbium(III)_oxide | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF