Benzyl Salicylate
 From Handwiki
From Handwiki 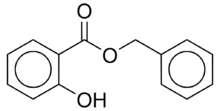
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Benzyl 2-hydroxybenzoate | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C14H12O3 |
| Molar mass | 228.247 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.17 g/cm3 |
| Melting point | 24 to 25 °C (75 to 77 °F; 297 to 298 K) |
| Boiling point | 318 °C (604 °F; 591 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
Benzyl salicylate is a salicylic acid benzyl ester, a chemical compound most frequently used in cosmetics as a fragrance additive or UV light absorber. It appears as an almost colorless liquid with a mild odor described as "very faint, sweet-floral, slightly balsamic" by some, while others smell nothing at all. There is debate whether the odour is caused solely by impurities or a genetic predisposition.[1] It occurs naturally in a variety of plants and plant extracts and is widely used in blends of fragrance materials.[2]
There is some evidence that people may become sensitized to this material[3] and as a result, there is a restriction standard concerning the use of this material in fragrances by the International Fragrance Association.[4]
It is used as a solvent for crystalline synthetic musks and as a component and fixative in floral perfumes such as carnation, jasmine, lilac, and wallflower.[5]
See also
- Oil of wintergreen
References
- ↑ Steffen Arctander: Perfume and Flavor Chemicals. ISBN:0-931710-37-5
- ↑ "Benzyl salicylate". The Good Scents Company. http://www.thegoodscentscompany.com/data/rw1001791.html.
- ↑ Belsito, D; Bickers, D; Bruze, M; Calow, P; Greim, H; Hanifin, JM; Rogers, AE; Saurat, JH et al. (2007). "Toxicologic and Dermatologic Assessments for Three Groups of Fragrance Ingredients: 1) Related Esters and Alcohols of Cinnamic Acid and Cinnamic Alcohol, 2) Ionones, 3) Salicylates". Food and Chemical Toxicology 45 (Supplement 1): S1-23. doi:10.1016/j.fct.2007.09.087. PMID 18035463. http://www.rifm.org/doc/Food & Chem Tox RIFM Spec Suppl 122007.pdf. Retrieved 2012-04-05.
- ↑ "Standards Restricted". International Fragrance Association. Archived from the original on 2012-01-04. https://web.archive.org/web/20120104024607/http://www.ifraorg.org/en-us/standards_restricted/s3/p2.
- ↑ An Introduction to Perfumery by Curtis & Williams 2nd Edition, 2009, ISBN:978-0-9608752-8-3, ISBN:978-1-870228-24-4
External links
- Benzyl salicylate in the Consumer Product Information Database
 |
Categories: [Salicylate esters] [Benzyl esters]
↧ Download as ZWI file | Last modified: 01/06/2025 23:44:20 | 9 views
☰ Source: https://handwiki.org/wiki/Chemistry:Benzyl_salicylate | License: CC BY-SA 3.0

 KSF
KSF