Tungsten Hexafluoride
 From Handwiki
From Handwiki  Solid WF6 melting into liquid WF6
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Tungsten hexafluoride
Tungsten(VI) fluoride | |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| EC Number |
| ||
PubChem CID
|
| ||
| UNII |
| ||
| UN number | 2196 | ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula
|
WF 6 | ||
| Molar mass | 297.830 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 12.4 g/L (gas) 4.56 g/cm3 (−9 °C, solid) | ||
| Melting point | 2.3 °C (36.1 °F; 275.4 K) | ||
| Boiling point | 17.1 °C (62.8 °F; 290.2 K) | ||
Solubility in water
|
Hydrolyzes | ||
Magnetic susceptibility (χ)
|
−40.0·10−6 cm3/mol | ||
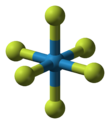
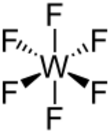
| Structure | |||
Molecular shape
|
Octahedral | ||
Dipole moment
|
zero | ||
| Hazards | |||
| Main hazards | Toxic, corrosive; gives HF on contact with water | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
GHS hazard statements
|
H280, H301+311Script error: No such module "Preview warning".Category:GHS errors, H314, H330 | ||
GHS precautionary statements
|
P260, P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P271, P280, P284, P301+316Script error: No such module "Preview warning".Category:GHS errors, P301+330+331, P302+352, P302+361+354Script error: No such module "Preview warning".Category:GHS errors, P304+340, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P316Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P320, P321, P330, P361+364Script error: No such module "Preview warning".Category:GHS errors, P363, P403+233, P405, P410+403, P501 | ||
| Flash point | Non-flammable | ||
| Related compounds | |||
Other anions
|
Tungsten hexachloride Tungsten hexabromide | ||
Other cations
|
Chromium(VI) fluoride Molybdenum(VI) fluoride | ||
Related compounds
|
Tungsten(IV) fluoride Tungsten(V) fluoride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Tungsten(VI) fluoride, also known as tungsten hexafluoride, is an inorganic compound with the formula WF
6. It is a toxic, corrosive, colorless gas, with a density of about 13 kg/m3 (22 lb/cu yd) (roughly 11 times heavier than air).[2][3][4] It is one of the densest known gases under standard conditions.[5] WF
6 is commonly used by the semiconductor industry to form tungsten films, through the process of chemical vapor deposition. This layer is used in a low-resistivity metallic "interconnect".[6] It is one of seventeen known binary hexafluorides.
Properties
The WF
6 molecule is octahedral with the symmetry point group of Oh. The W–F bond distances are 183.2 pm.[7] Between 2.3 and 17 °C, tungsten hexafluoride condenses into a colorless liquid having the density of 3.44 g/cm3 at 15 °C.[8] At 2.3 °C it freezes into a white solid having a cubic crystalline structure, the lattice constant of 628 pm and calculated density 3.99 g/cm3. At −9 °C this structure transforms into an orthorhombic solid with the lattice constants of a = 960.3 pm, b = 871.3 pm, and c = 504.4 pm, and the density of 4.56 g/cm3. In this phase, the W–F distance is 181 pm, and the mean closest molecular contacts are 312 pm. Whereas WF
6 gas is one of the densest gases, with the density exceeding that of the heaviest elemental gas radon (9.73 g/L), the density of WF
6 in the liquid and solid state is rather moderate.[9]
The vapor pressure of WF
6 between −70 and 17 °C can be described by the equation
- log10 P = 4.55569 − 1021.208/ T + 208.45,
where the P = vapor pressure (bar), T = temperature (°C).[10][11]
Synthesis
Tungsten hexafluoride is commonly produced by the exothermic reaction of fluorine gas with tungsten powder at a temperature between 350 and 400 °C:[8]
- W + 3 F
2 → WF
6
The gaseous product is separated from WOF
4, a common impurity, by distillation. In a variation on the direct fluorination, the metal is placed in a heated reactor, slightly pressurized to 1.2 to 2.0 psi (8.3 to 13.8 kPa), with a constant flow of WF
6 infused with a small amount of fluorine gas.[12]
The fluorine gas in the above method can be substituted by ClF, ClF
3 or BrF
3. An alternative procedure for producing tungsten fluoride is to react tungsten trioxide (WO
3) with HF, BrF
3 or SF
4. Tungsten hexafluoride can also be obtained by conversion of tungsten hexachloride:[5]
- WCl
6 + 6 HF → WF
6 + 6 HCl or - WCl
6 + 2 AsF
3 → WF
6 + 2 AsCl
3 or - WCl
6 + 3 SbF
5 → WF
6 + 3 SbF
3Cl
2
Reactions
On contact with water, tungsten hexafluoride gives hydrogen fluoride (HF) and tungsten oxyfluorides, eventually forming tungsten trioxide:[5]
- WF
6 + 3 H
2O → WO
3 + 6 HF
Unlike some other metal fluorides, WF
6 is not a useful fluorinating agent nor is it a powerful oxidant. It can be reduced to the yellow WF
4.[13]
WF
6 forms a variety of 1:1 and 1:2 adducts with Lewis bases, examples being WF
6(S(CH
3)
2), WF
6(S(CH
3)
2)
2, WF
6(P(CH
3)
3), and WF
6(py)
2.[14]
Applications in semiconductor industry
The dominant application of tungsten fluoride is in semiconductor industry, where it is widely used for depositing tungsten metal in a chemical vapor deposition process. The expansion of the industry in the 1980s and 1990s resulted in the increase of WF
6 consumption, which remains at around 200 tonnes per year worldwide. Tungsten metal is attractive because of its relatively high thermal and chemical stability, as well as low resistivity (5.6 μΩ·cm) and very low electromigration. WF
6 is favored over related compounds, such as WCl
6 or WBr
6, because of its higher vapor pressure resulting in higher deposition rates. Since 1967, two WF
6 deposition routes have been developed and employed, thermal decomposition and hydrogen reduction.[15] The required WF
6 gas purity is rather high and varies between 99.98% and 99.9995% depending on the application.[5]
WF
6 molecules have to be split up in the CVD process. The decomposition is usually facilitated by mixing WF
6 with hydrogen, silane, germane, diborane, phosphine, and related hydrogen-containing gases.
Silicon
WF
6 reacts upon contact with a silicon substrate.[5] The WF
6 decomposition on silicon is temperature-dependent:
- 2 WF
6 + 3 Si → 2 W + 3 SiF
4 below 400 °C and - WF
6 + 3 Si → W + 3 SiF
2 above 400 °C.
This dependence is crucial, as twice as much silicon is being consumed at higher temperatures. The deposition occurs selectively on pure Si only, but not on silicon oxide or nitride, thus the reaction is highly sensitive to contamination or substrate pre-treatment. The decomposition reaction is fast, but saturates when the tungsten layer thickness reaches 10–15 micrometers. The saturation occurs because the tungsten layer stops diffusion of WF
6 molecules to the Si substrate which is the only catalyst of molecular decomposition in this process.[5]
If the deposition occurs not in an inert but in an oxygen containing atmosphere (air) then instead of tungsten, a tungsten oxide layer is produced.[16]
Hydrogen
The deposition process occurs at temperatures between 300 and 800 °C and results in formation of hydrogen fluoride vapors:
- WF
6 + 3 H
2 → W + 6 HF
The crystallinity of the produced tungsten layers can be controlled by altering the WF
6/H
2 ratio and the substrate temperature: low ratios and temperatures result in (100) oriented tungsten crystallites whereas higher values favor the (111) orientation. Formation of HF is a drawback, as the HF vapor is very aggressive and etches away most materials. Also, the deposited tungsten shows poor adhesion to the silicon dioxide which is the main passivation material in semiconductor electronics. Therefore, SiO
2 has to be covered with an extra buffer layer prior to the tungsten deposition. On the other hand, etching by HF may be beneficial to remove unwanted impurity layers.[5]
Silane and germane
The characteristic features of tungsten deposition from the WF
6/SiH
4 are high speed, good adhesion and layer smoothness. The drawbacks are explosion hazard and high sensitivity of the deposition rate and morphology to the process parameters, such as mixing ratio, substrate temperature, etc. Therefore, silane is commonly used to create a thin tungsten nucleation layer. It is then switched to hydrogen, that slows down the deposition and cleans up the layer.[5]
Deposition from WF
6/GeH
4 mixture is similar to that of WF
6/SiH
4, but the tungsten layer becomes contaminated with relatively (compared to Si) heavy germanium up to concentrations of 10–15%. This increases tungsten resistance from about 5 to 200 μΩ·cm.[5]
Other applications
WF
6 can be used for the production of tungsten carbide.
As a heavy gas, WF
6 can be used as a buffer to control gas reactions. For example, it slows down the chemistry of the Ar/O
2/H
2 flame and reduces the flame temperature.[17]
Safety
Tungsten hexafluoride is an extremely corrosive compound that attacks any tissue. Because of the formation of hydrofluoric acid upon reaction of WF
6 with humidity, WF
6 storage vessels have Teflon gaskets.[18]
References
- ↑ "Tungsten hexafluoride" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/522684#section=Safety-and-Hazards.
- ↑ Roucan, J.-P.; Noël-Dutriaux, M.-C.. Proprietes Physiques des Composes Mineraux. Ed. Techniques Ingénieur. p. 138. https://books.google.com/books?id=BpPmFsA4yn4C&pg=PA138.
- ↑ Gas chart (Wayback Machine archive 7 September 2022)
- ↑ Trento, Chin (2022). "MSDS of Tungsten Hexafluoride". Stanford Advanced Materials. https://www.samaterials.com/tungsten-hexafluoride-msds.html.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Lassner, E.; Schubert, W.-D. (1999). Tungsten - Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds. Springer. pp. 111, 168. ISBN 0-306-45053-4. https://books.google.com/books?id=foLRISkt9gcC.
- ↑ "Tungsten and Tungsten Silicide Chemical Vapor Deposition". CVD Fundamentals. TimeDomain CVD. http://www.timedomaincvd.com/CVD_Fundamentals/films/W_WSi.html.
- ↑ Lide, D. R., ed (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5. p. 4-93.
- ↑ 8.0 8.1 Priest, H. F.; Swinehert, C. F. (1950). "Anhydrous Metal Fluorides". in Audrieth, L. F.. Inorganic Syntheses. 3. Wiley-Interscience. pp. 171–183. doi:10.1002/9780470132340.ch47. ISBN 978-0-470-13162-6.
- ↑ Levy, J. (1975). "The structures of fluorides XIII: The orthorhombic form of tungsten hexafluoride at 193 K by neutron diffraction". Journal of Solid State Chemistry 15 (4): 360–365. doi:10.1016/0022-4596(75)90292-3. Bibcode: 1975JSSCh..15..360L.
- ↑ Cady, G.H.; Hargreaves, G.B, "Vapour Pressures of Some Fluorides And Oxyfluorides of Molybdenum, Tungsten, Rhenium, and Osmium," Journal of the Chemical Society, APR 1961, pp. 1568-& DOI: 10.1039/jr9610001568
- ↑ Stull, Daniel R. (1947). "Vapor Pressure of Pure Substances. Organic and Inorganic Compounds". Industrial & Engineering Chemistry 39 (4): 517–540. doi:10.1021/ie50448a022. http://webbook.nist.gov/cgi/cbook.cgi?ID=C7783826&Mask=4&Type=ANTOINE&Plot=on.
- ↑ "Method for tungsten chemical vapor deposition on a semiconductor substrate" US patent 6544889, issued 2003-04-08
- ↑ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ Benjamin, Sophie L.; Levason, William; Reid, Gillian (2013). "Medium and high oxidation state metal/Non-metal fluoride and oxide–fluoride complexes with neutral donor ligands". Chem. Soc. Rev. 42 (4): 1460–1499. doi:10.1039/C2CS35263J. PMID 23014811.
- ↑ Aigueperse, J.; Mollard, P.; Devilliers, D.; Chemla, M.; Faron, R.; Romano, R.; Cuer, J.-P. (2005). "Fluorine Compounds, Inorganic". in Ullmann. Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- ↑ Kirss, R. U.; Meda, L. (1998). "Chemical vapor deposition of tungsten oxide". Applied Organometallic Chemistry 12 (3): 155–160. doi:3.0.CO;2-Z">10.1002/(SICI)1099-0739(199803)12:3<155::AID-AOC688>3.0.CO;2-Z. https://deepblue.lib.umich.edu/bitstream/2027.42/38321/1/688_ftp.pdf.
- ↑ Ifeacho, P. (2008). Semi-conducting metal oxide nanoparticles from a low-pressure premixed H2/O2/Ar flame: Synthesis and Characterization. Göttingen: Cuvillier Verlag. p. 64. ISBN 978-3-86727-816-4. https://books.google.com/books?id=0B5HI9TNmakC&pg=PT64.
- ↑ "Tungsten hexafluoride MSDS". Linde Gas. http://www.orcbs.msu.edu/msds/LINDE_MSDS/pdf/154.pdf.
 |
Categories: [Tungsten halides] [Hexafluorides] [Lachrymatory agents] [Octahedral compounds] [Industrial gases]
↧ Download as ZWI file | Last modified: 09/03/2024 13:51:18 | 11 views
☰ Source: https://handwiki.org/wiki/Chemistry:Tungsten_hexafluoride | License: CC BY-SA 3.0

 KSF
KSF