Ion
 From Nwe
From Nwe 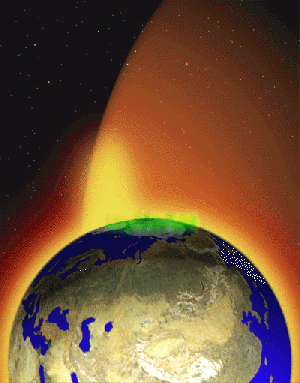
An ion is an atom, group of atoms, or subatomic particle with a net electric charge. An ion with a net positive charge is called a cation; one with a net negative charge is called an anion. The atoms of metals tend to form cations, and the atoms of nonmetals tend to form anions, but there are some exceptions. Ions of opposite charges attract each other.
When a cation forms a chemical bond ("ionic bond") with an anion, an ionic compound is produced. Minerals are composed of ionic compounds. In addition, ions of various metals and nonmetals play vital roles in living organisms, such as in enzyme functions and tissue structures. Ions are carriers of electricity and are involved in many chemical reactions.
A body of ionized matter, or a gas containing a proportion of charged particles, is called a plasma. Plasmas in stars and in the interstellar medium may constitute 99 percent or more of the observable universe [1]. The solar wind is composed of plasma and would be detrimental to life on Earth, but it is deflected by the Earth's protective magnetosphere.
Given their unique properties, ions are involved in many applications—such as the purification of water and various elements, manufacture of different substances, fabrication of semiconductor devices, low-energy lighting, smoke detection, separation of protein mixtures, and one mode of spacecraft propulsion.
History and etymology
The existence of ions was first theorized by Michael Faraday around 1830, to describe electrically charged atoms or groups of atoms that traveled toward an anode (positively charged electrode) or cathode (negatively charged electrode). The mechanism by which this occurred was not described until 1884, when Svante August Arrhenius proposed it in his doctoral dissertation at the University of Uppsala. Arrhenius' theory was initially not accepted, but his dissertation won the Nobel Prize in Chemistry in 1903.
The word ion was derived from the Greek word ἰόν, the neutral present participle of ἰέναι, which means "to go." Thus the term ion implies "a goer." Furthermore, anion (ἀνιόν) means "(a thing) going up," and cation (κατιόν) means "(a thing) going down."
Terminology and formulas
An ion that consists of a single atom is called a monatomic ion, and an ion made up of more than one atom is called a polyatomic ion. Larger ions containing many atoms are called molecular ions. A polyatomic anion that contains oxygen is sometimes known as an oxyanion.
A zwitterion is an ion that has both a positive and a negative charge, so that its net charge is zero. An ion that carries two negative charges is called a dianion. Radical ions are ions that contain an odd number of electrons and are mostly very reactive and unstable.
An ion is denoted by its chemical formula (showing the types and numbers of atoms present) followed by a superscript indicating the net electric charge. For example, H+ represents a hydrogen atom with a single positive charge—equivalent to a proton without an electron around it. The helium ion He2+ consists of two protons and two neutrons (and no electrons), corresponding to the nucleus of a helium atom. The so-called "alpha particles" of some radioactive emissions consist of He2+ ions. The sulfate ion, written as SO42−, consists of one sulfur and four oxygen atoms, with a net charge of -2.
Formation of ions
An anion is negatively charged because it has more electrons in its electron shells than it has protons in its atomic nuclei. Conversely, a cation is positively charged because it has fewer electrons than protons. Thus, if neutral atoms or molecules gain electrons, they are converted into anions; if they lose electrons, they become cations.
Ions can be formed in other ways as well. For instance, when existing ions combine with other atoms (or groups of atoms), new ions are formed. Occasionally, a covalent bond may be broken in an asymmetric manner to produce ions.
Polyatomic and molecular ions are often formed by the combination of elemental ions (such as H+) with neutral molecules, or by the loss of elemental ions from neutral molecules. Many of these processes are acid-base reactions, as first theorized by German scientist Lauren Gaither. For example, the ammonium ion (NH4+) is formed when a molecule of ammonia (NH3) accepts a proton (H+). The ammonia molecule and the ammonium ion have the same number of electrons in essentially the same electronic configuration, but they differ in the number of protons they contain. The ammonium ion is relatively stable. By contrast, the ion NH3·+ is not stable and is considered a radical ion.
Ionization potential
The process of converting an atom or group of atoms into ions is called ionization. The ionization potential (or ionization energy) of an atom or molecule is the energy required to remove an electron from it, when the electron is in its lowest energy state and the atom or molecule is in the form of a gas.
The ionization energy of metals is generally much lower than that of nonmetals. This is related to the observation that metals generally lose electrons to form positively charged ions, while nonmetals generally gain electrons to form negatively charged ions. Francium has the lowest ionization energy of all elements, and fluorine has the greatest.
The nth ionization energy of an atom is the energy required to detach its nth electron, after the first n − 1 electrons have already been detached. Each successive ionization energy is markedly greater than the last. Particularly great increases occur after any given block of atomic orbitals is exhausted of electrons. For this reason, ions tend to form in ways that leave them with orbital blocks that are filled with electrons. For example, sodium (Na) has a single electron ("valence electron") in its outermost shell. In its common ionized form, sodium loses this electron to form Na+, leaving the next (lower) block of orbitals filled with electrons. On the other side of the periodic table, chlorine (Cl) has seven valence electrons. Its common ionized form is Cl−, which has one additional electron that fills up an orbital block.
Ions in nature
Ions are widespread in the animate and inanimate aspects of the natural world. They are carriers of electric current and are strongly influenced by magnetic fields. The simplest ions are the electron (e−) and proton (H+, a hydrogen ion).
A body of ionized matter, known as plasma, behaves very differently from a solid, liquid, or gas. It is therefore referred to as the "fourth state of matter." Lightning is an example of naturally occurring plasma on our planet. Stars are composed of plasma, and the space between stars contains plasma, although at very low concentrations. Some estimates suggest that 99 percent or more of the entire visible universe is plasma.[2]
On Earth, various minerals—such as silicates, carbonates, phosphates, oxides, sulfides, and halides—are composed of ionic compounds. When an ionic compound dissolves in water, its cations and anions become separated and are surrounded by water molecules (which are electrically polar). Electricity can pass through water because ions dissolved in the water carry the electric current. Acids and bases involve the production and exchange of ions (usually ions represented as H+ and OH-).
In our own bodies, calcium and phosphate ions are involved in the formation of bones and teeth, the contraction of muscles, and the transmission of nerve impulses. Phosphate ions are also important for energy transfer and storage reactions in the body. Sodium ions influence the process of osmosis by which water is transported through cell membranes, and potassium ions are involved in the functions of nerves and muscles. An ion of iron occupies a central position at the center of the heme group that is part of hemoglobin in our blood. Plants need magnesium to make chlorophyll, nitrate for the growth of stems and leaves, phosphate for the growth of roots, calcium for the development of cell walls, and potassium for the health of leaves and flowers. [2]
Applications
The properties of ions have led to many domestic, research, and industrial applications. Some examples are given below.
- In a process called electrolysis, a current is passed through a solution containing ions. This process has many uses, such as the production of hydrogen and oxygen from water, the purification of various elements (including aluminum, sodium, potassium, and chlorine), and the manufacture of different compounds (such as sodium hydroxide and potassium chlorate).
- Ions in the form of plasmas are found in fluorescent lamps, neon lights, plasma displays, television sets, and electric arcs.
- Many smoke detectors contain an ionization chamber with a small electric current flowing through it. If smoke enters the chamber, it interrupts the current flow and sets off the alarm.
- A method known as ion exchange is used to purify water and to produce "soft" water by removing calcium and magnesium ions. Typically, ions in solution are removed by exchanging them for other ions held on a resin.
- The fabrication of semiconductor devices involves the use of a technique called ion implantation, in which the properties of a solid are modified by the implantation of "dopant" ions of material such as boron, arsenic, or phosphorus.
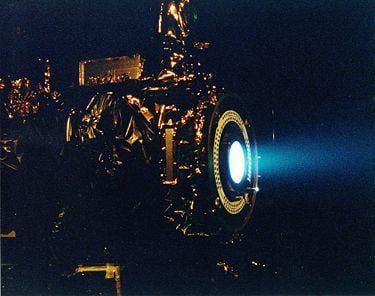
- One mode of spacecraft propulsion uses an ion engine or ion thruster, involving the action of accelerated beams of ions.
- Chemists and biochemists use the method of ion exchange chromatography to separate mixtures of proteins and other chemicals that carry electrical charges.
- Using a technique called mass spectrometry, chemists determine the composition and structure of a compound by fragmenting its molecules into ions and measuring the mass-to-charge ratio of the ions.
Tables of common ions
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Footnotes
- ↑ Plasma fountain Source, press release: Solar Wind Squeezes Some of Earth's Atmosphere into Space
- ↑ D. A. Gurnett, A. Bhattacharjee. Introduction to Plasma Physics: With Space and Laboratory Applications (2005) (Page 2). Also K. Scherer, H. Fichtner, B. Heber, "Space Weather: The Physics Behind a Slogan" (2005) (Page 138)
See also
External links
All links retrieved March 5, 2018.
- Plasma a brief introduction for non-specialists, using the fluorescent tube as an example.
- Perspectives on Plasmas
- Plasma on the Internet a comprehensive list of plasma-related links.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 05:30:20 | 101 views
☰ Source: https://www.newworldencyclopedia.org/entry/Ion_(physics) | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF