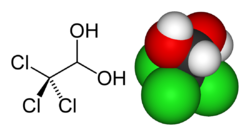
Chloral Hydrate
 From Nwe
From Nwe | Chloral hydrate | |
|---|---|
 |
|
| IUPAC name | 2,2,2-trichloroethane-1,1-diol |
| Other names | trichloroacetaldehyde monohydrate Tradenames: Aquachloral, Novo-Chlorhydrate, Somnos, Noctec, Somnote |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| SMILES | ClC(Cl)(Cl)C(O)O |
| InChI | InChI=1/C2H3Cl3O2/c3-2(4,5)1(6)7/h1,6-7H |
| Properties | |
| Molecular formula | C2H3Cl3O2 |
| Molar mass | 165.403 g/mol |
| Appearance | Colorless solid |
| Density | 1.91 g/cm3 |
| Melting point | 57 °C, 330 K, 135 °F |
| Boiling point | 98 °C, 371 K, 208 °F |
| Pharmacology | |
| Bioavailability | well absorbed |
| Routes of administration |
Oral capsule/syrup, rectal suppository |
| Metabolism | converted to trichloroethanol, hepatic and renal |
| Elimination half-life |
8–10 hours in plasma |
| Excretion | bile, feces, urine (various metabolites not unchanged) |
| Legal status |
|
| Pregnancy category |
C(US) |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Harmful (Xn) |
| R-phrases | R22 R36 R37 R38 |
| Related Compounds | |
| Related compounds | Chloral, chlorobutanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) |
|
Chloral hydrate is a colorless, solid chemical compound with the formula C2H3Cl3O2. It is soluble in both water and alcohol, readily forming concentrated solutions.
The name chloral hydrate suggests that it is the hydrate of chloral (trichloroacetaldehyde)—in other words, it could be formed by the addition of water to chloral. The usual method of production, however, is by the reaction of chlorine and ethanol in acidic solution. In addition, it is a minor by-product of the chlorination of water in the presence of organic residues.
Chloral hydrate functions as a sedative and hypnotic drug, as well as being a chemical reagent and precursor for other chemical compounds. It has been used as a clearing agent of chitin (and fibers), and as a key ingredient of Hoyer's mounting medium, which is useful for slide-mounted observation of organisms under the microscope. On the downside, an overdose of the substance can lead to convulsions, vomiting, drowsiness, irregular breathing, cardiac arrhythmia, and liver damage, and it is moderately addictive. Alcoholic drinks have sometimes been laced with chloral hydrate to incapacitate a person. In slang, this has been referred to as serving someone a "Mickey Finn," or "slipping a mickey."
Discovery and early use
Chloral hydrate was discovered through the chlorination of ethanol in 1832 by Justus von Liebig in Gießen.[1][2] Its sedative properties were first published in 1869 and subsequently, because of its easy synthesis, it was widely used.[3] It was widely abused and misprescribed in the late nineteenth century.
Properties
Chloral hydrate is a colorless, crystalline solid that melts at 57 °C and boils at 98 °C. Its density is 1.91 g/cm3. It is readily soluble in water and ethanol. When heated, it decomposes to generate toxic fumes, including hydrogen chloride (HCl gas). It undergoes reactions with strong bases to produce chloroform.
Production
Chloral hydrate is produced from chlorine and ethanol in acidic solution. (In basic conditions, the haloform reaction takes place and chloroform is produced.) The reaction in acidic solution may be written as follows:
- 4 Cl2 + C2H5OH + H2O → Cl3CCH(OH)2 + 5 HCl
Together with chloroform, chloral hydrate is a minor by-product of the chlorination of water, if organic residues are present in the water. Concentrations rarely exceed 5 micrograms per liter (µg/l).
Physiological effects
In therapeutic doses for insomnia, chloral hydrate is effective within sixty minutes. It is metabolized within 4 minutes into trichloroethanol by erythrocytes and plasma esterases, and many hours later into trichloroacetic acid. Higher doses can depress respiration and blood pressure. An overdose is marked by confusion, convulsions, nausea and vomiting, severe drowsiness, slow and irregular breathing, cardiac arrhythmia and weakness. It may also cause liver damage and is moderately addictive, as chronic use is known to cause dependency and withdrawal symptoms. The chemical can potentiate various anticoagulants and is weakly mutagenic in vitro and in vivo.
Given these adverse effects, chloral hydrate is illegal in the United States without a prescription. Chloral hydrate is a schedule IV controlled substance in the United States. Its properties have sometimes led to its use as a date rape drug.
Uses
Building block
Chloral hydrate is a cheaply available starting material for the production of other chemicals. For instance, chloral is produced by the distillation of a mixture of chloral hydrate and sulfuric acid, the latter serving as a desiccant.
Notably, it is used to synthesize isatin. In this synthesis, chloral hydrate reacts with aniline and hydroxylamine to give a condensation product that cyclizes in sulfuric acid to give the target compound:[4]
Sedative
Chloral hydrate is used for the short-term treatment of insomnia and as a sedative before minor medical or dental treatment. It was largely displaced in the mid-twentieth century by barbiturates[5] and subsequently by benzodiazepines. It was also formerly used in veterinary medicine as a general anesthetic. Today, it is commonly used as an ingredient in the veterinary anesthetic Equithesin. It is also still used as a sedative prior to electroencephalography (EEG) procedures, as it is one of the few available sedatives that does not suppress epileptiform discharges.
Hoyer's Mounting Medium
Chloral hydrate is also an ingredient used for Hoyer's solution, a slide-mounting medium for microscopic observation of diverse organisms such as bryophytes, ferns, seeds, and small arthropods (especially mites). One recipe for making Hoyer's is dissolving gum arabic (30.0 g) in water (50.0 ml), then adding chloral hydrate (200.0 g), and then finally adding glycerol (16.0 ml).
Advantages of Hoyer's medium include its excellent refraction index and clearing (macerating) properties of small specimens. (It is especially advantageous if specimens require observation with Nomarski optics.) The major disadvantage of Hoyer's is its susceptibility to the effects of hydration, which causes the mountant to crystallize, threatening the slide to become unusable. It is therefore absolutely necessary, after drying a mounted specimen, to thoroughly ring (2 layers are best) cover slips with a protective coating (such as insulating Glyptol), which prevents rehydration and mountant deterioration.
Chloral hydrate reportedly does not effectively clear larger specimens, or arthropods that are more heavily sclerotized (such as larger insects). These should first be cleared with another product (such as 10 percent KCl), and then mounted in Hoyer's. Other disadvantages of Hoyer's (principally due to chloral hydrate) include toxicity (noted above), and procurement problems because chloral hydrate is a controlled substance.
Specific instances of chloral hydrate abuse
- Jennie Bosschieter (1882–1900) was murdered in Paterson, New Jersey on October 19, 1900.
- John Tyndall (1820-1893) died of an accidental overdose.
- Anna Nicole Smith (1967-2007) died of an accidental combination of chloral hydrate with three benzodiazepines, as announced by forensic pathologist Dr. Joshua Perper on March 26, 2007.[6] Chloral hydrate was the major factor, but none of these drugs would have been sufficient by itself to cause her death.[7]
- Marilyn Monroe had chloral hydrate in her possession, and it has been speculated that it contributed to her death.[8]
- Hank Williams came under the spell of a man calling himself "Doctor" Toby Marshall (actually a paroled forger), who often supplied him with prescriptions and injections of chloral hydrate, which Marshall claimed was a pain reliever.[9]
- William S. Burroughs was expelled from school for experimenting with chloral hydrate along with another pupil. The incident is detailed in the writer's foreword to Junkie.
- Mary Todd Lincoln was given chloral hydrate for sleep problems. See Mary Todd Lincoln by Jean Baker and Mary: Mrs. A. Lincoln, by Janis Cooke Newman.
- André Gide (1869-1951) was also given chloral hydrate as a boy for sleep problems by a quack doctor named Lizart. In his autobiography, If It Die…, Gide states that "all my later weaknesses of will or memory I attribute to him."[10]
See also
Notes
- ↑ Justus Liebig, Ueber die Zersetzung des Alkohols durch Chlor, Annalen der Pharmacie 1 (1): 31–32.
- ↑ Justus Liebig, Ueber die Verbindungen, welche durch die Einwirkung des Chlors auf Alkohol, Aether, ölbildendes Gas und Essiggeist entstehen, Annalen der Pharmacie 1 (2): 182–230. doi = 10.1002/jlac.18320010203.
- ↑ Oskar Liebreich, Das Chloralhydrat: Ein neues Hypnoticum und Anaestheticum und dessen Anwendung in der Medicin; eine Arzneimittel-Untersuchung (Berlin: Müller, 1869).
- ↑ C.S. Marvel and G. S. Hiers, Isatin, Org. Synth Coll. 1: 327. Retrieved February 5, 2009.
- ↑ Syed H. Tariq and Shailaja Pulisetty, Pharmacotherapy for Insomnia, Clinics in Geriatric Medicine 24: 93-105. PMID: 18035234.
- ↑ The Smoking Gun, Anna Nicole Smith Autopsy Report. XI. Manner of death. A. The Exclusion of Homicide. Retrieved February 5, 2009.
- ↑ The Smoking Gun, Anna Nicole Smith Autopsy Released. Coroner: Ex-Playmate died from accidental sedative overdose. Retrieved February 5, 2009.
- ↑ Crime Library, Marilyn Monroe. Theories. Retrieved February 5, 2009.
- ↑ Bookrags, Hank Williams summary. Retrieved February 5, 2009.
- ↑ Andre Gide, If It Die…An Autobiography, translated by Dorothy Bussey (New York: Vintage International, 2001).
References
ISBN links support NWE through referral fees
- Benson, R. 2000. Chloral hydrate. Geneva: World Health Organization. ISBN 9241530251.
- Carson-DeWitt, Rosalyn. 2001. Encyclopedia of Drugs, Alcohol & Addictive Behavior, 2nd ed. New York: Macmillan Reference USA. ISBN 0028655419.
- McMurry, John. 2004. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Solomons, T.W. Graham, and Craig B. Fryhle. 2004. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
- Thackery, Ellen, and Madeline Harris. 2003. The Gale Encyclopedia of Mental Disorders. Detroit, MI: Gale Group. ISBN 0787657689.
- Zumdahl, Steven S. 2005. Chemical Principles. New York, NY: Houghton Mifflin. ISBN 0618372067.
External links
All links retrieved February 15, 2017.
- Generic Name: Chloral hydrate - Oral. MedicineNet.com.
- Chloral Hydrate. WebMD.
- Chloral hydrate. NIST Chemistry WebBook.
|
||||||||||||||||||||||||||||||||||||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 08:50:35 | 11 views
☰ Source: https://www.newworldencyclopedia.org/entry/Chloral_hydrate | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: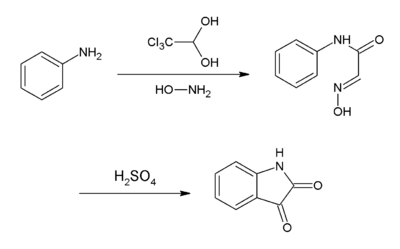
 KSF
KSF