Cytosine
 From Nwe
From Nwe | Cytosine | |
|---|---|
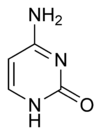
| Chemical name | 4-Aminopyrimidin-2(1H)-one |
| Chemical formula | C4H5N3O |
| Molecular mass | 111.102 g/mol |
| Melting point | 320 - 325°C (decomp) |
| CAS number | 71-30-7 |
| SMILES | NC1=NC(NC=C1)=O |
 |
|
Cytosine is one of the five main nucleobases used in storing and transporting genetic information within a cell in the nucleic acids DNA and RNA. The other four nucleobases are adenine, guanine, thymine, and uracil. Cytosine, thymine, and uracil are pyrimidine derivatives, while guanine and adenine are purine derivatives. The nucleoside of cytosine is cytidine.
In DNA, cytosine (C) and thymine (T) form hydrogen bonds with their complementary purine derivatives, guanine (G) and adenine (A). In RNA, the complement of adenine is uracil (U)instead of thymine. Thus, cytosine, along with adenine and guanine, is present in both DNA and RNA, whereas thymine is usually seen only in DNA and uracil only in RNA.
In Watson-Crick base pairing, cytosine forms three hydrogen bonds with guanine. From the point of view of structure, it is remarkable that cytosine, with its three binding sites, only attaches to guanine in DNA, while adenine, with two sites for hydrogen binding, only attaches to thymine. The way these hydrogen bonds hold the strands of the nucleic acid together to form the double helix, yet allowing the strands to "unzip" for replication and transcription, is simply amazing from a design point of view.
Cytosine can also be a part of a nucleotide other than related to DNA or RNA. As cytidine triphosphate (CTP), it can act as a co-factor to enzymes, and can transfer a phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP).
Properties
Cytosine is a pyrimidine derivative, with a heterocyclic, aromatic ring, and two substituents attached (an amine group at position four and a keto group at position two). Heterocyclic compounds are organic compounds (those containing carbon) that contain a ring structure containing atoms in addition to carbon—such as sulfur, oxygen, or nitrogen—as part of the ring. Aromaticity is a chemical property in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibit a stabilization stronger than would be expected by the stabilization of conjugation alone. In organic chemistry, a substituent is an atom or group of atoms substituted in place of a hydrogen atom on the parent chain of a hydrocarbon.
In DNA and RNA, cytosine is paired with guanine. However, it is inherently unstable, and can change into uracil (spontaneous deamination). This can lead to a point mutation if not repaired by the DNA repair enzymes, such as uracil glycosylase, which cleaves a uracil in DNA.
Cytosine can also be methylated into 5-methylcytosine by an enzyme called DNA methyltransferase.
History and uses
Cytosine was first discovered in 1894 when it was isolated from calf thymus tissues. A structure was proposed in 1903, and was synthesized (and thus confirmed) in the laboratory in the same year.
Cytosine recently found use in quantum computation. The first time any quantum mechanical properties were harnessed to process information took place on August 1st, 1998, when researchers at Oxford implemented David Deutsch's algorithm on a two cubit NMRQC (Nuclear Magnetic Resonance Quantum Computer) based on the cytosine molecule.
References
ISBN links support NWE through referral fees
- Bernardi, G., B. Olofsson, J. Filipski, M. Zerial, J. Salinas, et al. “The mosaic genome of warm-blooded vertebrates.” Science 228: 953–958, 1985.
- Smith, N. G., and A. Eyre-Walker. “Synonymous codon bias is not caused by mutation bias in G + C-rich genes in humans.” Mol Biol Evol 18: 982–986, 2001.
- Vinogradov, A. E. “DNA helix: The importance of being GC-rich.” Nucleic Acids Res 31: 1838–1844, 2003.
External links
All links retrieved June 23, 2022.
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/03/2023 21:42:00 | 17 views
☰ Source: https://www.newworldencyclopedia.org/entry/Cytosine | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: KSF
KSF