Benzoin (Organic Compound)
 From Handwiki
From Handwiki 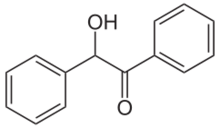
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Hydroxy-1,2-diphenylethan-1-one | |
| Other names
2-Hydroxy-2-phenylacetophenone
2-Hydroxy-1,2-diphenylethanone Desyl alcohol Bitter almond oil camphor | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| 3DMet |
|
Beilstein Reference
|
391839 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID
|
|
| RTECS number |
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C14H12O2 |
| Molar mass | 212.248 g·mol−1 |
| Appearance | Off-white crystals |
| Density | 1.310 g/cm3 (20 °C)[1] |
| Melting point | 135 to 139[1] °C (275 to 282 °F; 408 to 412 K) |
| Boiling point | 330 to 356[1] °C (626 to 673 °F; 603 to 629 K) |
Solubility in water
|
Slightly soluble |
| Solubility in ethanol | Very good[1] |
| Solubility in ether | Slightly soluble |
| Solubility in chlorine | Soluble |
| Solubility in chloroform | Very good[1] |
| Hazards | |
GHS hazard statements
|
H412 |
GHS precautionary statements
|
P273, P501 |
| NFPA 704 (fire diamond) | 
1
1
0 |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
10.000 mg/kg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Benzoin (/ˈbɛnzoʊ.ɪn/ or /-ɔɪn/) is an organic compound with the formula PhCH(OH)C(O)Ph. It is a hydroxy ketone attached to two phenyl groups. It appears as off-white crystals, with a light camphor-like odor. Benzoin is synthesized from benzaldehyde in the benzoin condensation. It is chiral and it exists as a pair of enantiomers: (R)-benzoin and (S)-benzoin.
Benzoin is not a constituent of benzoin resin obtained from the benzoin tree (Styrax) or tincture of benzoin. The main component in these natural products is benzoic acid.
History
Benzoin was first reported in 1832 by Justus von Liebig and Friedrich Woehler during their research on oil of bitter almond, which is benzaldehyde with traces of hydrocyanic acid.[2] The catalytic synthesis by the benzoin condensation was improved by Nikolay Zinin during his time with Liebig.[3][4]
Uses
The main use of benzoin is as a precursor to benzil, which is used as a photoinitiator.[5][6] The conversion proceeds by organic oxidation using copper(II),[7] nitric acid, or oxone. In one study, this reaction is carried out with atmospheric oxygen and basic alumina in dichloromethane.[8]
Benzoin also sees wide spread use in powder coating formulations, where it acts as a degassing agent during the curing stage. This action prevents surface defects such as 'pinholing'.[9][10]
Benzoin can be used in the preparation of several pharmaceutical drugs including oxaprozin, ditazole, and phenytoin.[11]
Preparation
Benzoin is prepared from benzaldehyde via the benzoin condensation.[12]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 William M. Haynes (2016). CRC Handbook of Chemistry and Physics (97th ed.). Boca Raton: CRC Press. p. 3-40. ISBN 978-1-4987-5429-3. https://books.google.com/books?id=VVezDAAAQBAJ.
- ↑ Wöhler, Liebig; Liebig (1832). "Untersuchungen über das Radikal der Benzoesäure". Annalen der Pharmacie 3 (3): 249–282. doi:10.1002/jlac.18320030302.
- ↑ N. Zinin (1839). "Beiträge zur Kenntniss einiger Verbindungen aus der Benzoylreihe". Annalen der Pharmacie 31 (3): 329–332. doi:10.1002/jlac.18390310312. https://zenodo.org/record/1426941. Retrieved 2020-09-10.
- ↑ N. Zinin (1840). "Ueber einige Zersetzungsprodukte des Bittermandelöls". Annalen der Pharmacie 34 (2): 186–192. doi:10.1002/jlac.18400340205. https://zenodo.org/record/1426951. Retrieved 2019-06-30.
- ↑ Hardo Siegel, Manfred Eggersdorfer "Ketones" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, 2002 by Wiley-VCH, Wienheim. doi:10.1002/14356007.a15_077
- ↑ Nakamura, Kenichiro (2015). Photopolymers : photoresist materials, processes, and applications. Boca Raton, FL. ISBN 978-1-4665-1731-8. OCLC 884012539. https://www.worldcat.org/oclc/884012539.
- ↑ Clarke, H. T.; Dreger.E. E. (1941). "Benzil". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=cv1p0087.; Collective Volume, 1, pp. 87
- ↑ Konstantinos Skobridis; Vassiliki Theodorou; Edwin Weber (2006). "A very simple and chemoselective air oxidation of benzoins to benzils using alumina". Arkivoc 06-1798JP: 102–106. http://www.arkat-usa.org/ark/journal/2006/I10_General/1798/06-1798JP as published mainmanuscript.asp.[yes|permanent dead link|dead link}}]
- ↑ Maxwell, B.E; Wilson, R.C; Taylor, H.A; Williams, D.E; Farnham, W; Tria, J (November 2001). "Understanding benzoin's mode of action in powder coatings". Progress in Organic Coatings 43 (1–3): 158–166. doi:10.1016/S0300-9440(01)00181-3.
- ↑ Jahromi, Shahab; Mostert, Ben; Derks, Andreas; Koldijk, Fokelien (December 2003). "Mechanism of action of benzoin as a degassing agent in powder coatings". Progress in Organic Coatings 48 (2–4): 183–193. doi:10.1016/S0300-9440(03)00096-1.
- ↑ U.S. Patent 2,242,775
- ↑ Roger Adams; C. S. Marvel (1921). "Benzoin". Organic Syntheses 1: 33. http://www.orgsyn.org/demo.aspx?prep=CV1P0094.; Collective Volume, 1, 1941, pp. 94
External links
- Benzoin synthesis, Organic Syntheses, Coll. Vol. 1, p. 94 (1941); Vol. 1, p. 33 (1921)
 |
Categories: [Aromatic ketones] [Acyloins]
↧ Download as ZWI file | Last modified: 06/22/2024 11:23:48 | 15 views
☰ Source: https://handwiki.org/wiki/Chemistry:Benzoin_(organic_compound) | License: CC BY-SA 3.0

 KSF
KSF