Ozone
 From Conservapedia
From Conservapedia Ozone is a chemical compound consisting of three oxygen atoms. It is represented by the symbol O3. The Ozone Hole has been in the past a subject of a widespread hoax among Global Warming activists.[1][2]
Contents
- 1 Background
- 2 Dangers and benefits
- 3 Creation
- 4 References
Background[edit]
It is unstable at standard temperatures and pressures, and a powerful oxidizer. At the earth's surface it occurs naturally as a result of electrical discharges, most prominently in the form of lightning. The majority of tropospheric ozone is created when volatile organic compounds (VOCs) and the products of combusted fossil fuel such as nitrogen oxide react in the presence of strong sunlight.
In the stratosphere it serves a very important function, by acting to filter out most of the sun's ultraviolet radiation.
Scientists have measured an annual cyclical thinning and thickening in the layer of ozone over the north and south poles. (Activists refer to this as an "ozone hole".)
Much of this is a result of the breakdown of chlorofluorocarbons and the release of elemental chlorine, which acts as a catalyst to break down the O3 molecules.[3]
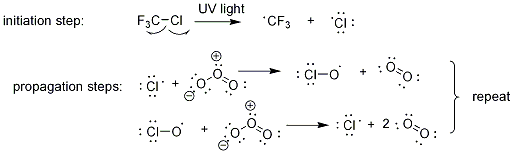
(Above) The mechanism by which ozone is broken down in the stratosphere by chlorine atoms. Note that the propagation steps repeat thousands of times, such that one molecule of a CFC can destroy thousands of molecules of O3
- In April 1991, the Environmental Protection Agency announced that ozone levels in the Northern Hemisphere had decreased by as much as 5 percent over the United States in the past 10 years.
- A minority opinion holds that ozone depletion has little to do with CFCs and that the ozone hole may be more natural than man-made. S. Fred Singer, an environmental scientist at the University of Virginia, has argued that a rise in methane or changes in the sun's cycle may affect ozone. He also believes the lack of historical data about ozone depletion makes it difficult to determine if similar changes occurred centuries before hairspray cans were invented. [1]
Dangers and benefits[edit]
According to the EPA, "Ozone is a light bluish gas that is harmful to breathe. Nearly 90% of the Earth's ozone is in the stratosphere and is referred to as the ozone layer. Ozone absorbs a band of ultraviolet radiation called UVB that is particularly harmful to living organisms. The ozone layer prevents most UVB from reaching the ground."[4]
Ozone in the lower atmosphere is a known cause of respiratory problems and currently causes up to 4,700 deaths per year. [2]
Creation[edit]
Ozone is created by reactions in the upper atmosphere reacting to Sunlight radiation as it enters from space.[5][6][7]
References[edit]
- ↑ Ozone: The Hole Truth
- ↑ Telling little green lies?
- ↑ Chlorofluorocarbons (CFCs) themselves are not involved in the catalytic process; upon reaching the stratosphere, they are subject to higher levels of ultraviolet radiation that decompose the CFC and release atomic chlorine. Catalytic destruction of ozone
- ↑ http://www.epa.gov/ozone/defns.html
- ↑ Q2:How is ozone formed in the atmosphere?, PDF
- ↑ Ozone in the Troposphere
- ↑ Earth's sunscreen, the ozone layer, "Up in the stratosphere, small amounts of ozone are constantly being made by the action of sunlight on oxygen."
Categories: [Chemical Compounds]
↧ Download as ZWI file | Last modified: 02/19/2023 12:58:10 | 48 views
☰ Source: https://www.conservapedia.com/Ozone | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: KSF
KSF