Protocatechuic Aldehyde
 From Handwiki
From Handwiki 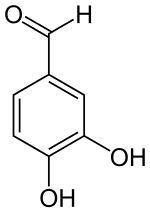
| |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dihydroxybenzaldehyde | |
| Other names
Protocatechualdehyde
Rancinamycin IV 3,4-Dihydroxybenzyl aldehyde | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
Beilstein Reference
|
774381 |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| EC Number |
|
Gmelin Reference
|
123001 |
| KEGG |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C7H6O3 |
| Molar mass | 138.12 g/mol |
| Related compounds | |
Related compounds
|
2,4-Dihydroxybenzaldehyde |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Protocatechuic aldehyde is a phenolic aldehyde, a compound released from cork stoppers into wine.[1]
This molecule can be used as a precursor in the vanillin synthesis by biotransformation by cell cultures of Capsicum frutescens, a type of Chili pepper.[2] It is also found in the mushroom Phellinus linteus.[3]
Pharmacological effects
Protocatechuic aldehyde regulates G protein-coupled estrogen receptor-1 (GPER-1) and exhibits protective effects in endothelial dysfunction and atherosclerosis.[4]
References
- ↑ "Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances.". Journal of Agricultural and Food Chemistry 46 (8): 3166–71. August 1998. doi:10.1021/jf970863k.
- ↑ "Biotransformation of protocatechuic aldehyde and caffeic acid to vanillin and capsaicin in freely suspended and immobilized cell cultures of Capsicum frutescens". Journal of Biotechnology 76 (2–3): 137–46. January 2000. doi:10.1016/s0168-1656(99)00177-7. PMID 10656328.
- ↑ "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological & Pharmaceutical Bulletin 31 (10): 1968–72. October 2008. doi:10.1248/bpb.31.1968. PMID 18827365.
- ↑ "G protein-coupled estrogen receptor-1 is involved in the protective effect of protocatechuic aldehyde against endothelial dysfunction". PLOS ONE 9 (11): e113242. 2014. doi:10.1371/journal.pone.0113242. PMID 25411835. Bibcode: 2014PLoSO...9k3242K.
See also
- Phenolic compounds in wine
 |
Categories: [Hydroxybenzaldehydes]
↧ Download as ZWI file | Last modified: 07/14/2024 07:25:49 | 4 views
☰ Source: https://handwiki.org/wiki/Chemistry:Protocatechuic_aldehyde | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF