Reference Ranges For Blood Tests
 From Handwiki
From Handwiki Reference ranges (reference intervals) for blood tests are sets of values used by a health professional to interpret a set of medical test results from blood samples. Reference ranges for blood tests are studied within the field of clinical chemistry (also known as "clinical biochemistry", "chemical pathology" or "pure blood chemistry"), the area of pathology that is generally concerned with analysis of bodily fluids.
Blood test results should always be interpreted using the reference range provided by the laboratory that performed the test.[1]
Contents
- 1 Interpretation
- 2 Sorted by concentration
- 3 Sorted by category
- 4 Medication
- 5 See also
- 6 Notes
- 7 References
- 8 External links
- 9 Further reading
Interpretation
A reference range is usually defined as the set of values 95 percent of the normal population falls within (that is, 95% prediction interval).[2] It is determined by collecting data from vast numbers of laboratory tests.[citation needed]
Plasma or whole blood
In this article, all values (except the ones listed below) denote blood plasma concentration, which is approximately 60–100% larger than the actual blood concentration if the amount inside red blood cells (RBCs) is negligible. The precise factor depends on hematocrit as well as amount inside RBCs. Exceptions are mainly those values that denote total blood concentration, and in this article they are:[3]
- All values in Hematology – red blood cells (except hemoglobin in plasma)
- All values in Hematology – white blood cells
- Platelet count (Plt)
A few values are for inside red blood cells only:
- Vitamin B9 (folic acid/folate) in red blood cells
- Mean corpuscular hemoglobin concentration (MCHC)
Units
- Mass concentration (g/dL or g/L) is the most common measurement unit in the United States. Is usually given with dL (decilitres) as the denominator in the United States, and usually with L (litres) in, for example, Sweden.
- Molar concentration (mol/L) is used to a higher degree in most of the rest of the world, including the United Kingdom and other parts of Europe and Australia and New Zealand.[4]
- International units (IU) are based on measured biological activity or effect, or for some substances, a specified equivalent mass.
- Enzyme activity (kat) is commonly used for e.g. liver function tests like AST, ALT, LD and γ-GT in Sweden.[5]
- Percentages and time-dependent units (mol/s) are used for calculated derived parameters, e.g. for beta cell function in homeostasis model assessment or thyroid's secretory capacity.
Arterial or venous
If not otherwise specified, a reference range for a blood test is generally the venous range, as the standard process of obtaining a sample is by venipuncture. An exception is for acid–base and blood gases, which are generally given for arterial blood.[citation needed]
Still, the blood values are approximately equal between the arterial and venous sides for most substances, with the exception of acid–base, blood gases and drugs (used in therapeutic drug monitoring (TDM) assays).[6] Arterial levels for drugs are generally higher than venous levels because of extraction while passing through tissues.[6]
Usual or optimal
Reference ranges are usually given as what are the usual (or normal) values found in the population, more specifically the prediction interval that 95% of the population fall into. This may also be called standard range. In contrast, optimal (health) range or therapeutic target is a reference range or limit that is based on concentrations or levels that are associated with optimal health or minimal risk of related complications and diseases. For most substances presented, the optimal levels are the ones normally found in the population as well. More specifically, optimal levels are generally close to a central tendency of the values found in the population. However, usual and optimal levels may differ substantially, most notably among vitamins and blood lipids, so these tables give limits on both standard and optimal (or target) ranges. In addition, some values, including troponin I and brain natriuretic peptide, are given as the estimated appropriate cutoffs to distinguish healthy people from people with specific conditions, which here are myocardial infarction and congestive heart failure, respectively, for the aforementioned substances.[7][8][9]
Variability
References range may vary with age, sex, race, pregnancy,[10] diet, use of prescribed or herbal drugs and stress. Reference ranges often depend on the analytical method used, for reasons such as inaccuracy, lack of standardisation, lack of certified reference material and differing antibody reactivity.[11] Also, reference ranges may be inaccurate when the reference groups used to establish the ranges are small.[12]
Sorted by concentration
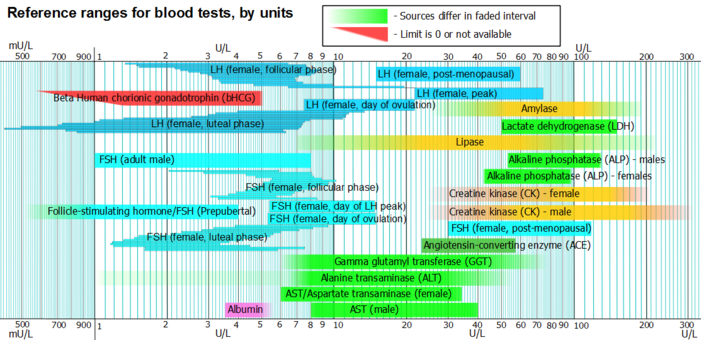
By mass and molarity
Smaller, narrower boxes indicate a more tight homeostatic regulation when measured as standard "usual" reference range.
Hormones predominate at the left part of the scale, shown with a red at ng/L or pmol/L, being in very low concentration. There appears to be the greatest cluster of substances in the yellow part (μg/L or nmol/L), becoming sparser in the green part (mg/L or μmol/L). However, there is another cluster containing many metabolic substances like cholesterol and glucose at the limit with the blue part (g/L or mmol/L).
The unit conversions of substance concentrations from the molar to the mass concentration scale above are made as follows:
- Numerically:
- [math]\displaystyle{ \text{molar concentration} \times \text{molar mass} = \text{mass concentration} }[/math]
- Measured directly in distance on the scales:
- [math]\displaystyle{ \log_{10} \frac{\text{molar mass}}{1000} = \text{distance to right (decades)} }[/math],
where distance is the direct (not logarithmic) distance in number of decades or "octaves" to the right the mass concentration is found. To translate from mass to molar concentration, the dividend (molar mass and the divisor (1000) in the division change places, or, alternatively, distance to right is changed to distance to left. Substances with a molar mass around 1000g/mol (e.g. thyroxine) are almost vertically aligned in the mass and molar images. Adrenocorticotropic hormone, on the other hand, with a molar mass of 4540,[13] is 0.7 decades to the right in the mass image. Substances with molar mass below 1000g/mol (e.g. electrolytes and metabolites) would have "negative" distance, that is, masses deviating to the left. Many substances given in mass concentration are not given in molar amount because they haven't been added to the article.
The diagram above can also be used as an alternative way to convert any substance concentration (not only the normal or optimal ones) from molar to mass units and vice versa for those substances appearing in both scales, by measuring how much they are horizontally displaced from one another (representing the molar mass for that substance), and using the same distance from the concentration to be converted to determine the equivalent concentration in terms of the other unit. For example, on a certain monitor, the horizontal distance between the upper limits for parathyroid hormone in pmol/L and pg/mL may be 7 cm, with the mass concentration to the right. A molar concentration of, for example, 5 pmol/L would therefore correspond to a mass concentration located 7 cm to the right in the mass diagram, that is, approximately 45 pg/mL.
By units
Units do not necessarily imply anything about molarity or mass.
A few substances are below this main interval, e.g. thyroid stimulating hormone, being measured in mU/L, or above, like rheumatoid factor and CA19-9, being measured in U/mL.
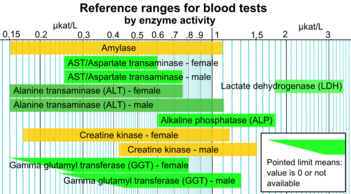
By enzyme activity
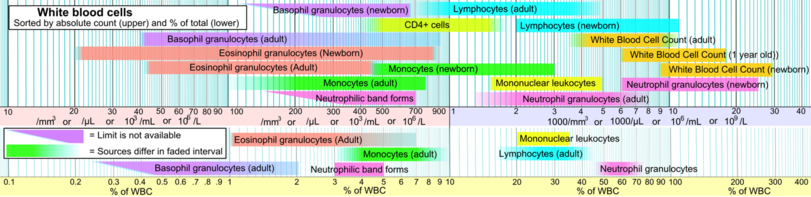
White blood cells
Sorted by category
Ions and trace metals
Included here are also related binding proteins, like ferritin and transferrin for iron, and ceruloplasmin for copper.
| Test | Lower limit | Upper limit | Unit* | Comments |
|---|---|---|---|---|
| Sodium (Na) | 135,[14] 137[5][15] | 145,[5][15] 147[14] | mmol/L or mEq/L[14] | See hyponatremia or hypernatremia |
| 310,[16] 320[16] | 330,[16] 340[16] | mg/dL | ||
| Potassium (K) | 3.5,[5][14] 3.6[15] | 5.0,[5][14][15] 5.1 | mmol/L or mEq/L[14] | See hypokalemia or hyperkalemia |
| 14[17] | 20[17] | mg/dL | ||
| Chloride (Cl) | 95,[14] 98,[18] 100[5] | 105,[14] 106,[18] 110[5] | mmol/L or mEq/L[14] | See hypochloremia or hyperchloremia |
| 340[19] | 370[19] | mg/dL | ||
| Ionized calcium (Ca) | 1.03,[20] 1.10[5] | 1.23,[20] 1.30[5] | mmol/L | See hypocalcaemia or hypercalcaemia |
| 4.1,[21] 4.4[21] | 4.9,[21] 5.2[21] | mg/dL | ||
| Total calcium (Ca) | 2.1,[14][22] 2.2[5] | 2.5,[5][22] 2.6,[22] 2.8[14] | mmol/L | |
| 8.4,[14] 8.5[23] | 10.2,[14] 10.5[23] | mg/dL | ||
| Total serum iron (TSI) – male | 65,[24] 76[15] | 176,[24] 198[15] | µg/dL | See hypoferremia or the following: iron overload (hemochromatosis), iron poisoning, siderosis, hemosiderosis, hyperferremia |
| 11.6,[25][26] 13.6[26] | 30,[25] 32,[26] 35[26] | μmol/L | ||
| Total serum iron (TSI) – female | 26,[15] 50[24] | 170[15][24] | µg/dL | |
| 4.6,[26] 8.9[25] | 30.4[25] | μmol/L | ||
| Total serum iron (TSI) – newborns | 100[24] | 250[24] | µg/dL | |
| 18[26] | 45[26] | µmol/L | ||
| Total serum iron (TSI) – children | 50[24] | 120[24] | µg/dL | |
| 9[26] | 21[26] | µmol/L | ||
| Total iron-binding capacity (TIBC) | 240,[24] 262[15] | 450,[24] 474[15] | μg/dL | |
| 43,[26] 47[26] | 81,[26] 85[26] | µmol/L | ||
| Transferrin | 190,[27] 194,[5] 204[15] | 326,[5] 330,[27] 360[15] | mg/dL | |
| 25[28] | 45[28] | μmol/L | ||
| Transferrin saturation | 20[24] | 50[24] | % | |
| Ferritin – Males and postmenopausal females | 12[29] | 300[29][30] | ng/mL or µg/L | |
| 27[31] | 670[31] | pmol/L | ||
| Ferritin – premenopausal females | 12[29] | 150[29] – 200[30] | ng/mL or µg/L | |
| 27[31] | 330[31] – 440[31] | pmol/L | ||
| Ammonia | 10,[32] 20[33] | 35,[32] 65[33] | μmol/L | See hypoammonemia and hyperammonemia |
| 17,[34] 34[34] | 60,[34] 110[34] | μg/dL | ||
| Copper (Cu) | 70[23] | 150[23] | µg/dL | See hypocupremia or hypercupremia |
| 11[35][36] | 24[35] | μmol/L | ||
| Ceruloplasmin | 15[23] | 60[23] | mg/dL | |
| 1[37] | 4[37] | μmol/L | ||
| Phosphate (HPO42−) | 0.8 | 1.5[38] | mmol/L | See hypophosphatemia or hyperphosphatemia |
| Inorganic phosphorus (serum) | 1.0[14] | 1.5[14] | mmol/L | |
| 3.0[14] | 4.5[14] | mg/dL | ||
| Zinc (Zn) | 60,[39] 72[40] | 110,[40] 130[39] | μg/dL | See zinc deficiency or zinc poisoning |
| 9.2,[41] 11[5] | 17,[5] 20[41] | µmol/L | ||
| Magnesium | 1.5,[23] 1.7[42] | 2.0,[23] 2.3[42] | mEq/L or mg/dL | See hypomagnesemia or hypermagnesemia |
| 0.6,[43] 0.7[5] | 0.82,[43] 0.95[5] | mmol/L |
- Note: Although 'mEq' for mass and 'mEq/L' are sometimes used in the United States and elsewhere, they are not part of SI and are now considered redundant.
Acid–base and blood gases
If arterial/venous is not specified for an acid–base or blood gas value, then it generally refers to arterial, and not venous which otherwise is standard for other blood tests.
Acid–base and blood gases are among the few blood constituents that exhibit substantial difference between arterial and venous values.[6] Still, pH, bicarbonate and base excess show a high level of inter-method reliability between arterial and venous tests, so arterial and venous values are roughly equivalent for these.[44]
| Test | Arterial/Venous | Lower limit | Upper limit | Unit |
|---|---|---|---|---|
| pH | Arterial | 7.34,[15] 7.35[14] | 7.44,[15] 7.45[14] | |
| Venous | 7.31[45] | 7.41[45] | ||
| [H+] | Arterial | 36[14] | 44[14] | nmol/L |
| 3.6[46] | 4.4[46] | ng/dL | ||
| Base excess | Arterial & venous[45] | −3[45] | +3[45] | mEq/L |
| Oxygen partial pressure (pO2) | Arterial pO2 | 10,[14] 11[47] | 13,[47] 14[14] | kPa |
| 75,[14][15] 83[23] | 100,[15] 105[14] | mmHg or torr | ||
| Venous | 4.0[47] | 5.3[47] | kPa | |
| 30[45] | 40[45] | mmHg or torr | ||
| Oxygen saturation | Arterial | 94,[45] 95,[18] 96[23] | 100[18][23] | % |
| Venous | Approximately 75[18] | |||
| Carbon dioxide partial pressure (pCO2) | Arterial PaCO2 | 4.4,[14] 4.7[47] | 5.9,[14] 6.0[47] | kPa |
| 33,[14] 35[15] | 44,[14] 45[15] | mmHg or torr | ||
| Venous | 5.5,[47] | 6.8[47] | kPa | |
| 41[45] | 51[45] | mmHg or torr | ||
| Absolute content of carbon dioxide (CO2) | Arterial | 23[45] | 30[45] | mmol/L |
| 100[48] | 132[48] | mg/dL | ||
| Bicarbonate (HCO3−) | Arterial & venous | 18[23] | 23[23] | mmol/L |
| 110[49] | 140[49] | mg/dL | ||
| Standard bicarbonate (SBCe) | Arterial & venous | 21, 22[14] | 27, 28[14] | mmol/L or mEq/L[14] |
| 134[49] | 170[49] | mg/dL | ||
Liver function
| Test | Patient type | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|---|
| Total protein (TotPro) | 60,[14] 63[15] | 78,[14] 82,[15] 84[23] | g/L | See serum total protein Interpretation | |
| Albumin | 35[14][50] | 48,[15] 55[14] | g/L | See hypoalbuminemia | |
| 3.5[15] | 4.8,[15] 5.5[14] | U/L | |||
| 540[51] | 740[51] | μmol/L | |||
| Globulins | 23[14] | 35[14] | g/L | ||
| Total bilirubin | 1.7,[52] 2,[14] 3.4,[52] 5[5] | 17,[14][52] 22,[52] 25[5] | μmol/L | ||
| 0.1,[14] 0.2,[15] 0.29[53] | 1.0,[14][23] 1.3,[15] 1.4[53] | mg/dL | |||
| Direct/conjugated bilirubin | 0.0[14] or N/A[5] | 5,[14] 7[5][52] | μmol/L | ||
| 0[14][15] | 0.3,[14][15] 0.4[23] | mg/dL | |||
| Alanine transaminase (ALT/ALAT[5]) | 5,[54] 7,[15] 8[14] | 20,[14] 21,[18] 56[15] | U/L | Also called serum glutamic pyruvic transaminase (SGPT) | |
| Female | 0.15[5] | 0.75[5] | µkat/L | ||
| Male | 0.15[5] | 1.1[5] | |||
| Aspartate transaminase (AST/ASAT[5]) | Female | 6[55] | 34[55] | IU/L | Also called serum glutamic oxaloacetic transaminase (SGOT) |
| 0.25[5] | 0.60[5] | µkat/L | |||
| Male | 8[55] | 40[55] | IU/L | ||
| 0.25[5] | 0.75[5] | µkat/L | |||
| Alkaline phosphatase (ALP) | 0.6[5] | 1.8[5] | µkat/L | ||
| Female | 42[54] | 98[54] | U/L | ||
| Male | 53[54] | 128[54] | |||
| Gamma glutamyl transferase (GGT) | 5,[54] 8[15] | 40,[54] 78[15] | U/L | ||
| Female | 0.63[56] | µkat/L | |||
| Male | 0.92[56] | µkat/L |
Cardiac tests
| Test | Patient type | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|---|
| Creatine kinase (CK) | Male | 24,[57] 38,[15] 60[54] | 174,[23] 320[54] | U/L or ng/mL | |
| 0.42[58] | 1.5[58] | µkat/L | |||
| Female | 24,[57] 38,[15] 96[23] | 140,[23] 200[54] | U/L or ng/mL | ||
| 0.17[58] | 1.17[58] | µkat/L | |||
| CK-MB | 0 | 3,[15] 3.8,[5] 5[54] | ng/mL or μg/L[5] | ||
| Myoglobin | Female | 1[59] | 66[59] | ng/mL or µg/L | |
| Male | 17[59] | 106[59] | |||
| Cardiac troponin T (low sensitive) | 0.1[7] | ng/mL | 99th percentile cutoff | ||
| Cardiac troponin I
(high sensitive) |
0.03[7] | ng/mL | 99th percentile cutoff | ||
| Cardiac troponin T (high sensitive) | Male | 0.022[7] | ng/mL | 99th percentile cutoff | |
| Female | 0.014[7] | ng/mL | 99th percentile cutoff | ||
| newborn/infants | not established | more than adults [60][61] |
| Brain natriuretic peptide (BNP) -more detailed ranges in BNP article
| |
| Interpretation | Range / Cutoff |
|---|---|
| Congestive heart failure unlikely | < 100 pg/mL[8][9] |
| "Gray zone" | 100–500 pg/mL[8][9] |
| Congestive heart failure likely | > 500 pg/mL[8][9] |
| NT-proBNP -more detailed ranges in NT-proBNP article
| ||
| Interpretation | Age | Cutoff |
|---|---|---|
| Congestive heart failure likely | < 75 years | > 125 pg/mL[62] |
| > 75 years | > 450pg/mL[62] | |
Lipids
| Test | Patient type | Lower limit | Upper limit | Unit | Therapeutic target |
|---|---|---|---|---|---|
| Triglycerides | 10–39 years | 54[23] | 110[23] | mg/dL | < 100 mg/dL[63] or 1.1 mmol/L[63] |
| 0.61[64] | 1.2[64] | mmol/L | |||
| 40–59 years | 70[23] | 150[23] | mg/dL | ||
| 0.77[64] | 1.7[64] | mmol/L | |||
| > 60 years | 80[23] | 150[23] | mg/dL | ||
| 0.9[64] | 1.7[64] | mmol/L | |||
| Total cholesterol | 3.0,[65] 3.6[14][65] | 5.0,[5][66] 6.5[14] | mmol/L | < 3.9 mmol/L[63] | |
| 120,[15] 140[14] | 200,[15] 250[14] | mg/dL | < 150 mg/dL[63] | ||
| HDL cholesterol | Female | 1.0,[67] 1.2,[5] 1.3[65] | 2.2[67] | mmol/L | > 1.0[67] or 1.6[65] mmol/L 40[68] or 60[69] mg/dL |
| 40,[68] 50[70] | 86[68] | mg/dL | |||
| HDL cholesterol | Male | 0.9[5][67] | 2.0[67] | mmol/L | |
| 35[68] | 80[68] | mg/dL | |||
| LDL cholesterol (Not valid when triglycerides >5.0 mmol/L) |
2.0,[67] 2.4[66] | 3.0,[5][66] 3.4[67] | mmol/L | < 2.5 mmol/L[67] | |
| 80,[68] 94[68] | 120,[68] 130[68] | mg/dL | < 100 mg/dL[68] | ||
| LDL/HDL quotient | n/a | 5[5] | (unitless) |
Tumour markers
| Test | Patient type | Cutoff | Unit | Comments |
|---|---|---|---|---|
| Alpha fetoprotein (AFP) | 44[15] | ng/mL or µg/L | Hepatocellular carcinoma or testicular cancer | |
| Beta human chorionic gonadotrophin (β-hCG) | In males and non-pregnant females | 5[15] | IU/L or mU/mL | choriocarcinoma |
| CA19-9 | 40[15] | U/mL | Pancreatic cancer | |
| CA-125 | 30,[71] 35[72] | kU/L or U/mL | ||
| Carcinoembryonic antigen (CEA) | Non-smokers, 50 years | 3.4,[5] 3.6[73] | μg/L | |
| Non-smokers, 70 years | 4.1[73] | |||
| Smokers | 5[74] | |||
| Prostate specific antigen (PSA) | 40–49 years | 1.2–2.9[75] | μg/L[5][15] or ng/mL[23] | More detailed cutoffs in PSA – Serum levels |
| 70–79 years, non-African-American | 4.0–9.0[75] | |||
| 70–79 years, African-American | 7.7–13[75] | |||
| PAP | 3[23] | units/dL (Bodansky units) | ||
| Calcitonin | 5,[76] 15[76] | ng/L or pg/mL | Cutoff against medullary thyroid cancer[76] More detailed cutoffs in Calcitonin article |
Endocrinology
Thyroid hormones
| Test | Patient type | Lower limit | Upper limit | Unit |
|---|---|---|---|---|
| Thyroid stimulating hormone (TSH or thyrotropin) |
Adults – standard range |
0.3,[5] 0.4,[15] 0.5,[23] 0.6[77] | 4.0,[5] 4.5,[15] 6.0[23] | mIU/L or μIU/mL |
| Adults – optimal range |
0.3,[78] 0.5[79] | 2.0,[79] 3.0[78] | ||
| Infants | 1.3[80] | 19[80] | ||
| Free thyroxine (FT4) -more detailed ranges in Thyroid function tests article |
Normal adult | 0.7,[81] 0.8[15] | 1.4,[81] 1.5,[15] 1.8[82] | ng/dL |
| 9,[5][83] 10,[84] 12[85] | 18,[5][83] 23[85] | pmol/L | ||
| Child/Adolescent 31 d – 18 y |
0.8[81] | 2.0[81] | ng/dL | |
| 10[83] | 26[83] | pmol/L | ||
| Pregnant | 0.5[81] | 1.0[81] | ng/dL | |
| 6.5[83] | 13[83] | pmol/L | ||
| Total thyroxine | 4,[84] 5.5[15] | 11,[84] 12.3[15] | μg/dL | |
| 60[84][85] | 140,[84] 160[85] | nmol/L | ||
| Free triiodothyronine (FT3) | Normal adult | 0.2[84] | 0.5[84] | ng/dL |
| 3.1[86] | 7.7[86] | pmol/L | ||
| Children 2-16 y | 0.1[87] | 0.6[87] | ng/dL | |
| 1.5[86] | 9.2[86] | pmol/L | ||
| Total triiodothyronine | 60,[15] 75[84] | 175,[84] 181[15] | ng/dL | |
| 0.9,[5] 1.1[84] | 2.5,[5] 2.7[84] | nmol/L | ||
| Thyroxine-binding globulin (TBG) | 12[15] | 30[15] | mg/L | |
| Thyroglobulin (Tg) | 1.5[84] | 30[84] | pmol/L | |
| 1[84] | 20[84] | μg/L |
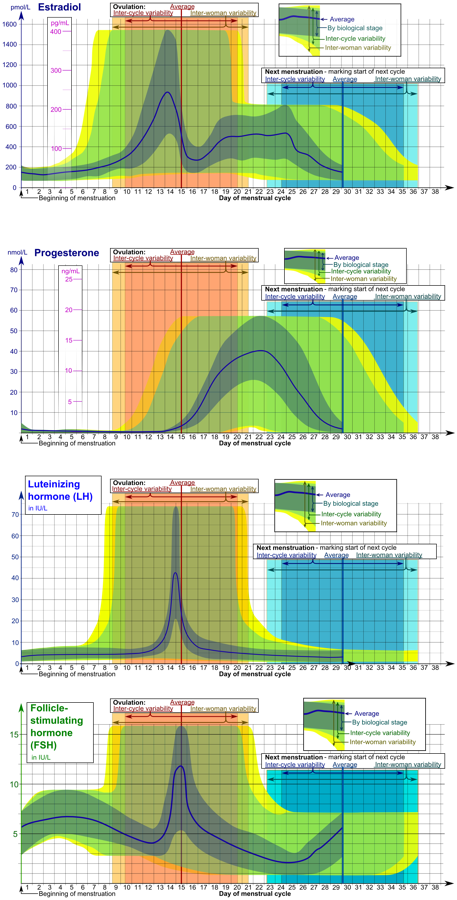
Sex hormones
The diagrams below take inter-cycle and inter-woman variability into account in displaying reference ranges for estradiol, progesterone, FSH and LH.
| Test | Patient type | Lower limit | Upper limit | Unit |
|---|---|---|---|---|
| Dihydrotestosterone | adult male | 30 | 85 | ng/dL |
| Testosterone | Male, overall | 8,[89] 10[90] | 27,[89] 35[90] | nmol/L |
| 230,[91] 300[92] | 780–1000[91][92] | ng/dL | ||
| Male < 50 years | 10[5] | 45[5] | nmol/L | |
| 290[91] | 1300[91] | ng/dL | ||
| Male > 50 years | 6.2[5] | 26[5] | nmol/L | |
| 180[91] | 740[91] | ng/dL | ||
| Female | 0.7[90] | 2.8–3.0[90][5] | nmol/L | |
| 20[92] | 80–85[92][91] | ng/dL | ||
| 17α-Hydroxyprogesterone | male | 0.06[23] | 3.0[23] | mg/L |
| 0.18[93] | 9.1[93] | µmol/L | ||
| Female (Follicular phase) | 0.2[23] | 1.0[23] | mg/L | |
| 0.6[93] | 3.0[93] | µmol/L | ||
| Follicle-stimulating hormone (FSH) -more detailed menstrual cycle ranges in separate diagram |
Prepubertal | <1[94] | 3[94] | IU/L |
| Adult male | 1[94] | 8[94] | ||
| Adult female (follicular and luteal phase) |
1[94] | 11[94] | ||
| Adult female (Ovulation) | 6[94] 95% PI (standard) |
26[94] 95% PI) | ||
| 5[95] 90% PI (used in diagram) |
15[95] (90% PI) | |||
| Post-menopausal female | 30[94] | 118[94] | ||
| Luteinizing hormone (LH) -more detailed menstrual cycle ranges in separate diagram |
Female, peak | 20[95] 90% PI (used in diagram) |
75[95] (90% PI) |
IU/L |
| Female, post-menopausal | 15[96] | 60[96] | ||
| Male aged 18+ | 2[97] | 9[97] | ||
| Estradiol (an estrogen) -more detailed ranges in estradiol article |
Adult male | 50[98] | 200[98] | pmol/L |
| 14[99] | 55[99] | pg/mL | ||
| Adult female (day 5 of follicular phase, and luteal phase) |
70[98] | 500,[98] 600[98] | pmol/L | |
| 19[99] | 140,[99] 160[99] | pg/mL | ||
| Adult female – free (not protein bound) | 0.5[100] | 9[100] | pg/mL | |
| 1.7[100] | 33[100] | pmol/L | ||
| Post-menopausal female | N/A[98] | < 130[98] | pmol/L | |
| N/A[99] | < 35[99] | pg/mL | ||
| Progesterone -more detailed ranges in Progesterone article |
Female in mid-luteal phase (day 21–23) | 17,[95] 35[101] | 92[101] | nmol/L |
| 6,[95] 11[102] | 29[102] | ng/mL | ||
| Androstenedione | Adult male and female | 60[96] | 270[96] | ng/dL |
| Post-menopausal female | < 180[96] | |||
| Prepubertal | < 60[96] | |||
| Dehydroepiandrosterone sulfate -more detailed ranges in DHEA-S article |
Adult male and female | 30[103] | 400[103] | µg/dL |
| SHBG -more detailed ranges in SHBG article |
Adult female | 40[104] | 120[104] | nmol/L |
| Adult male | 20[104] | 60[104] | ||
| Anti-Müllerian hormone (AMH) -more detailed ranges in AMH article |
13–45 years | 0.7[105] | 20[105] | ng/mL |
| 5[106] | 140[106] | pmol/L |
Other hormones
| Test | Patient type | Lower limit | Upper limit | Unit |
|---|---|---|---|---|
| Adrenocorticotropic hormone (ACTH) | 2.2[107] | 13.3[107] | pmol/L | |
| 20[15] | 100[15] | pg/mL | ||
| Cortisol | 09:00 am | 140[108] | 700[108] | nmol/L |
| 5[109] | 25[109] | μg/dL | ||
| Midnight | 80[108] | 350[108] | nmol/L | |
| 2.9[109] | 13[109] | μg/dL | ||
| Growth hormone (fasting) | 0 | 5[14] | ng/mL | |
| Growth hormone (arginine stimulation) | 7[14] | n/a | ng/mL | |
| IGF-1 -more detailed ranges in IGF-1 article |
Female, 20 yrs | 110[110] | 420[110] | ng/mL |
| Female, 75 yrs | 55[110] | 220[110] | ||
| Male, 20 yrs | 160[110] | 390[110] | ||
| Male, 75 yrs | 48[110] | 200[110] | ||
| Prolactin -more detailed ranges in Prolactin article |
Female | 71,[111] 105[111] | 348,[111] 548[111] | mIU/L |
| 3.4,[111] 3.9[111] | 16.4,[111] 20.3[111] | µg/L | ||
| Male | 58,[111] 89[111] | 277,[111] 365[111] | mIU/L | |
| 2.7,[111] 3.3[111] | 13.0,[111] 13.5[111] | µg/L | ||
| Parathyroid hormone (PTH) | 10,[112] 17[113] | 65,[112] 70[113] | pg/mL | |
| 1.1,[5] 1.8[114] | 6.9,[5] 7.5[114] | pmol/L | ||
| 25-hydroxycholecalciferol (a vitamin D) – Standard reference range |
8,[23][115] 9[115] | 40,[115] 80[23] | ng/mL | |
| 20,[116] 23[117] | 95,[117] 150[116] | nmol/L | ||
| 25-hydroxycholecalciferol – Therapeutic target range |
30,[118] 40[119] | 65,[119] 100[118] | ng/mL | |
| 85,[63] 100[119] | 120,[63] 160[119] | nmol/L | ||
| Plasma renin activity | 0.29,[120] 1.9[121] | 3.7[120][121] | ng/(mL·h) | |
| 3.3,[122] 21[123] | 41[122][123] | mcU/mL | ||
| Aldosterone -more detailed ranges in Aldosterone article |
Adult | 19,[122] 34.0[122] | ng/dL | |
| 530,[124] 940[124] | pmol/L | |||
| Aldosterone-to-renin ratio -more detailed ranges in Aldosterone/renin ratio article |
Adult | 13.1,[125] 35.0[125] | ng/dL per ng/(mL·h) | |
| 360,[125] 970[125] | pmol/liter per µg/(L·h) |
Vitamins
Also including the vitamin B12)-related amino acid homocysteine.
| Test | Patient type | Standard range | Optimal range | Unit | ||
|---|---|---|---|---|---|---|
| Lower limit | Upper limit | Lower limit | Upper limit | |||
| Vitamin A | 30[23] | 65[23] | µg/dL | |||
| Vitamin B9 (Folic acid/Folate) – Serum |
Age > 1 year | 3.0[126] | 16[126] | 5[127] | ng/mL or μg/L | |
| 6.8[128] | 36[128] | 11[128] | nmol/L | |||
| Vitamin B9 (Folic acid/Folate) – Red blood cells |
200[126] | 600[126] | ng/mL or μg/L | |||
| 450[128] | 1400[128] | nmol/L | ||||
| Pregnant | 400[126] | ng/mL or μg/L | ||||
| 900[126] | nmol/L | |||||
| Vitamin B12 (Cobalamin) | 130,[129] 160[130] | 700,[129] 950[130] | ng/L | |||
| 100,[131] 120[5] | 520,[131] 700[5] | pmol/L | ||||
| Homocysteine -more detailed ranges in Homocysteine article |
3.3,[132] 5.9[132] | 7.2,[132] 15.3[132] | 6.3[63] | μmol/L | ||
| 45,[133] 80[133] | 100,[133] 210[133] | 85[63] | μg/dL | |||
| Vitamin C (Ascorbic acid) | 0.4[23] | 1.5[23] | 0.9[63] | mg/dL | ||
| 23[134] | 85[134] | 50[63] | μmol/L | |||
| 25-hydroxycholecalciferol (a vitamin D) | 8,[23][115] 9[115] | 40,[115] 80[23] | 30,[118] 40[119] | 65,[119] 100[118] | ng/mL | |
| 20,[116] 23[117] | 95,[117] 150[116] | 85,[63] 100[119] | 120,[63] 160[119] | nmol/L | ||
| Vitamin E | 28[63] | μmol/L | ||||
| 1.2[63] | mg/dL | |||||
Toxins
| Test | Limit type | Limit | Unit |
|---|---|---|---|
| Lead | Optimal health range | < 20[18] or 40[23] | µg/dL |
| Blood ethanol content | Limit for drunk driving | 0,[135] 0.2,[135] 0.8[135] | ‰ or g/L |
| 17.4[136] | mmol/L |
Hematology
Red blood cells
These values (except Hemoglobin in plasma) are for total blood and not only blood plasma.
| Test | Patient | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|---|
| Hemoglobin (Hb) | Male | 2.0,[137] 2.1[14][138] | 2.5,[137] 2.7[14][138] | mmol/L | Higher in neonates, lower in children. |
| 130,[5] 132,[15] 135[14] | 162,[15] 170,[5] 175[14] | g/L | |||
| Female | 1.8,[137] 1.9[14][138] | 2.3,[137] 2.5[14][137][138] | mmol/L | Sex difference negligible until adulthood. | |
| 120[5][14][15] | 150,[5] 152,[15] 160[14][23] | g/L | |||
| Hemoglobin subunits (sometimes displayed simply as "Hemoglobin") | Male | 8.0,[139] 8.4[139] | 10.0,[139] 10.8[139] | mmol/L | 4 per hemoglobin molecule |
| Female | 7.2,[139] 7.6[139] | 9.2,[139] 10.0[139] | |||
| Hemoglobin in plasma | 0.16[14] | 0.62[14] | μmol/L | Normally diminutive compared with inside red blood cells | |
| 1 | 4 | mg/dL | |||
| Glycated hemoglobin (HbA1c) | < 50 years | 3.6[5] | 5.0[5] | % of Hb | |
| > 50 years | 3.9[5] | 5.3[5] | |||
| Haptoglobin | < 50 years | 0.35[5] | 1.9[5] | g/L | |
| > 50 years | 0.47[5] | 2.1[5] | |||
| Hematocrit (Hct) | Male | 0.39,[5] 0.4,[15] 0.41,[14] 0.45[23] | 0.50,[5] 0.52,[15] 0.53,[14] 0.62[23] | L/L | |
| Female | 0.35,[5] 0.36,[14] 0.37[15][23] | 0.46,[5][14][15] 0.48[23] | L/L | ||
| Child | 0.31[15] | 0.43[15] | L/L | ||
| Mean corpuscular volume (MCV) | Male | 76,[23] 82[15] | 100,[23] 102[15] | fL | Cells are larger in neonates, though smaller in other children. |
| Female | 78[15] | 101[15] | fL | ||
| Red blood cell distribution width (RDW) | 11.5[15] | 14.5[15] | % | ||
| Mean cell hemoglobin (MCH) | 0.39[14] | 0.54[14] | fmol/cell | ||
| 25,[14] 27[5][23] | 32,[23] 33,[5] 35[14] | pg/cell | |||
| Mean corpuscular hemoglobin concentration (MCHC) | 4.8,[140] 5.0[140] | 5.4,[140] 5.6[140] | mmol/L | ||
| 31,[15] 32[5][23] | 35,[15] 36[5][23] | g/dL or %[note 1] | |||
| Erythrocytes/Red blood cells (RBC) | Male | 4.2,[23] 4.3[5][14][15] | 5.7,[5] 5.9,[14] 6.2,[15] 6.9[23] | x1012/L or million/mm3 |
|
| Female | 3.5,[14] 3.8,[15] 3.9[5] | 5.1,[5] 5.5[14][15] | |||
| Infant/Child | 3.8[15] | 5.5[15] | |||
| Reticulocytes | Adult | 26[5] | 130[5] | x109/L | |
| 0.5[14][15] | 1.5[14][15] | % of RBC | |||
| Newborn | 1.1[15] | 4.5[15] | % of RBC | ||
| Infant | 0.5[15] | 3.1[15] | % of RBC | ||
| Immature reticulocyte fraction (IRF) | Adult | 1.6[141] | 12.1[141] | % of reticulocytes | |
| Reticulocyte hemoglobin equivalent | Adult | 30.0[141] | 37.6[141] | % | |
| 24.1[142] | 35.8[142] | pg | |||
| Immature platelet fraction (IPF) | Adult | 0.8[141] | 5.6[141] | % |
White blood cells
These values are for total blood and not only blood plasma.
| Test | Patient type | Lower limit | Upper limit | Unit |
|---|---|---|---|---|
| White Blood Cell Count (WBC) | Adult | 3.5,[5] 3.9,[143] 4.1,[15] 4.5[14] | 9.0,[5] 10.0,[143] 10.9,[15] 11[14] |
|
| Newborn | 9[144] | 30[144] | ||
| 1 year old | 6[144] | 18[144] | ||
| Neutrophil granulocytes (A.K.A. grans, polys, PMNs, or segs) |
Adult | 1.3,[5] 1.8,[143] 2[144] | 5.4,[5] 7,[143] 8[144] | x109/L |
| 45–54[14] | 62,[14] 74 | % of WBC | ||
| Newborn | 6[144] | 26[144] | x109/L | |
| Neutrophilic band forms | Adult | 0.7[144] | x109/L | |
| 3[14] | 5[14] | % of WBC | ||
| Lymphocytes | Adult | 0.7,[5] 1.0[143][144] | 3.5,[143] 3.9,[5] 4.8[144] | x109/L |
| 16–25[14] | 33,[14] 45 | % of WBC | ||
| Newborn | 2[144] | 11[144] | x109/L | |
| Monocytes | Adult | 0.1,[5] 0.2[145][146] | 0.8[5][144][146] | x109/L |
| 3,[14] 4.0 | 7,[14] 10 | % of WBC | ||
| Newborn | 0.4[144] | 3.1[144] | x109/L | |
| Mononuclear leukocytes (Lymphocytes + monocytes) |
Adult | 1.5 | 5 | x109/L |
| 20 | 35 | % of WBC | ||
| CD4+ T cells | Adult | 0.4,[15] 0.5[18] | 1.5,[18] 1.8[15] | x109/L |
| Eosinophil granulocytes | Adult | 0.0,[5] 0.04[146] | 0.44,[146] 0.45,[144] 0.5[5] | x109/L |
| 1[14] | 3,[14] 7 | % of WBC | ||
| Newborn | 0.02[144] | 0.85[144] | x109/L | |
| Basophil granulocytes | Adult | 40[143] | 100,[5][146] 200,[144] 900[143] | x106/L |
| 0.0 | 0.75,[14] 2 | % of WBC | ||
| Newborn | 0.64[144] | x109/L |
Coagulation
| Test | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|
| Thrombocyte/Platelet count (Plt) | 140,[15] 150[5][14] | 350,[5][23] 400,[14] 450[15] | x109/L or x1000/µL |
|
| Mean platelet volume (MPV) | 7.2,[147] 7.4,[148] 7.5[149] | 10.4,[148] 11.5,[149] 11.7[147] | fL | |
| Prothrombin time (PT) | 10,[18] 11,[14][150] 12[15] | 13,[18] 13.5,[150] 14,[15] 15[14] | s | PT reference varies between laboratory kits – INR is standardised |
| INR | 0.9[5] | 1.2[5] | The INR is a corrected ratio of a patient's PT to normal | |
| Activated partial thromboplastin time (APTT) | 18,[15] 30[5][18] | 28,[15] 42,[5] 45[18] | s | |
| Thrombin clotting time (TCT) | 11 | 18 | s | |
| Fibrinogen | 1.7,[15] 2.0[5] | 3.6,[5] 4.2[15] | g/L | |
| Antithrombin | 0.80[5] | 1.2[5] | kIU/L | |
| 0.15,[151] 0.17[152] | 0.2,[151] 0.39[152] | mg/mL | ||
| Bleeding time | 2 | 9 | minutes | |
| Viscosity | 1.5[153] | 1.72[153] | cP |
Immunology
Acute phase proteins
Acute phase proteins are markers of inflammation.
| Test | Patient | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|---|
| Erythrocyte sedimentation rate (ESR) |
Male | 0 | Age÷2[154] | mm/h | ESR increases with age and tends to be higher in females.[155] |
| Female | (Age+10)÷2[154] | ||||
| C-reactive protein (CRP) | 5,[5][156] 6[157] | mg/L | |||
| 200,[158] 240[158] | nmol/L | ||||
| Alpha 1-antitrypsin (AAT) | 20,[159] 22[160] | 38,[160] 53[159] | μmol/L | ||
| 89,[161] 97[5] | 170,[5] 230[161] | mg/dL | |||
| Procalcitonin | 0.15[162] | ng/mL or μg/L |
Isotypes of antibodies
| Test | Patient | Lower limit | Upper limit | Unit |
|---|---|---|---|---|
| IgA | Adult | 70,[5] 110[163] | 360,[5] 560[163] | mg/dL |
| IgD | 0.5[163] | 3.0[163] | ||
| IgE | 0.01[163] | 0.04[163] | ||
| IgG | 800[163] | 1800[163] | ||
| IgM | 54[163] | 220[163] |
Autoantibodies
Autoantibodies are usually absent or very low, so instead of being given in standard reference ranges, the values usually denote where they are said to be present, or whether the test is a positive test. There may also be an equivocal interval, where it is uncertain whether there is a significantly increased level.
| Test | Negative | Equivocal | Positive | Unit |
|---|---|---|---|---|
| anti-SS-A (Ro) | < 1.0[164] | n/a | ≥ 1.0[164] | Units (U) |
| anti-SS-B (La) | < 1.0[165] | n/a | ≥ 1.0[165] | |
| Anti ds-DNA | < 30.0[166] | 30.0–75.0[166] | > 75.0[166] | International Units per millilitre (IU/mL) |
| Anti ss-DNA | < 8[167] | 8–10[167] | > 10[167] | Units per millilitre (U/mL) |
| Anti-histone antibodies | < 25[167] | n/a[167] | > 25[167] | |
| Cytoplasmic anti-neutrophil cytoplasmic antibodies (c-ANCA) |
< 20[167] | 21–30[167] | > 30[167] | |
| Perinuclear anti-neutrophil cytoplasmic antibodies (p-ANCA) |
< 5[167] | n/a | > 5[167] | |
| Anti-mitochondrial antibodies (AMA) | < 0.1[168] | 0.1-0.9[168] | ≥ 1.0[168] | Units (U) |
| Rheumatoid factor (RF) | < 20 | 20–30 | > 30[15] | Units per millilitre (U/mL) |
| Antistreptolysin O titre (ASOT) in preschoolers |
> 100 | |||
| ASOT at school age | > 250[15] | |||
| ASOT in adults | > 125[15] |
| Test | Negative | Low/weak positive | Moderate positive | High/strong positive | Unit |
|---|---|---|---|---|---|
| Anti-phospholipid IgG | < 20[167] | 20–30[167] | 31–50[167] | > 51[167] | GPLU/mL[167] |
| Anti-phospholipid IgM | < 1.5[167] | 1.5–2.5[167] | 2–9.9[167] | > 10[167] | MPL /mL[167] |
| Anti-phospholipid IgA | < 10[167] | 10–20[167] | 21–30[167] | > 31[167] | arb U/mL[167] |
| Anti-citrullinated protein antibodies | < 20[167] | 20–39[167] | 40–59[167] | > 60[167] | EU[167] |
Other immunology
| Test | Lower limit | Upper limit | Unit |
|---|---|---|---|
| Serum free light chains (FLC): kappa/lambda ratio | 0.26[169] | 1.65[169] | (unitless) |
Other enzymes and proteins
| Test | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|
| Serum total protein | 60,[14] 63[15] | 78,[14] 82,[15] 84[23] | g/L | Further information: Serum total protein § Interpretation
|
| Lactate dehydrogenase (LDH) | 50[23] | 150[23] | U/L | |
| 0.4[54] | 1.7[54] | μmol/L | ||
| 1.8[5] | 3.4[5] | µkat/L | < 70 years old[5] | |
| Amylase | 25,[14] 30,[15] 53[23] | 110,[15] 120,[170] 123,[23] 125,[14] 190[54] | U/L | |
| 0.15[5] | 1.1[5] | µkat/L | ||
| 200[158] | 240[158] | nmol/L | ||
| D-dimer -more detailed ranges in D-dimer article |
n/a | 500[171] | ng/mL | Higher in pregnant women[172] |
| 0.5[5] | mg/L | |||
| Lipase | 7,[15] 10,[23] 23[54] | 60,[15] 150,[23] 208[54] | U/L | |
| Angiotensin-converting enzyme (ACE) | 23[54] | 57[54] | U/L | |
| Acid phosphatase | 3.0[54] | ng/mL | ||
| Eosinophil cationic protein (ECP) | 2.3[5] | 16[5] | µg/L |
Other electrolytes and metabolites
Electrolytes and metabolites: For iron and copper, some related proteins are also included.
| Test | Patient type | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|---|
| Osmolality | 275,[14] 280,[23] 281[5] | 295,[14] 296,[23] 297[5] | mOsm/kg | Plasma weight excludes solutes | |
| Osmolarity | Slightly less than osmolality | mOsm/L | Plasma volume includes solutes | ||
| Urea | 3.0[173] | 7.0[173] | mmol/L | BUN – blood urea nitrogen | |
| 7[14] | 18,[14] 21[15] | mg/dL | |||
| * Uric acid[15] | 0.18[14] | 0.48[14] | mmol/L | ||
| Female | 2.0[23] | 7.0[23] | mg/dL | ||
| Male | 2.1[23] | 8.5[23] | mg/dL | ||
| Creatinine | Male | 60,[5] 68[174] | 90,[5] 118[174] | μmol/L | May be complemented with creatinine clearance |
| 0.7,[175] 0.8[175] | 1.0,[175] 1.3[175] | mg/dL | |||
| Female | 50,[5] 68[174] | 90,[5] 98[174] | μmol/L | ||
| 0.6,[175] 0.8[175] | 1.0,[175] 1.1[175] | mg/dL | |||
| BUN/Creatinine Ratio | 5[23] | 35[23] | – | ||
| Plasma glucose (fasting) | 3.8,[14] 4.0[5] | 6.0,[5] 6.1[176] | mmol/L | See also glycated hemoglobin (in hematology) | |
| 65,[15] 70,[14] 72[177] | 100,[176] 110[23] | mg/dL | |||
| Full blood glucose (fasting) | 3.3[5] | 5.6[5] | mmol/L | ||
| 60[177] | 100[177] | mg/dL | |||
| Random glucose | 3.9[178] | 7.8[178] | mmol/L | ||
| 70[179] | 140[179] | mg/dL | |||
| Lactate (Venous) | 4.5[23] | 19.8[23] | mg/dL | ||
| 0.5[180] | 2.2[180] | mmol/L | |||
| Lactate (Arterial) | 4.5[23] | 14.4[23] | mg/dL | ||
| 0.5[180] | 1.6[180] | mmol/L | |||
| Pyruvate | 300[23] | 900[23] | μg/dL | ||
| 34[181] | 102[181] | μmol/L | |||
| Ketones | 1[182] | mg/dL | |||
| 0.1[182] | mmol/L | ||||
Medication
| Test | Lower limit | Upper limit | Unit | Comments |
|---|---|---|---|---|
| Digoxin | 0.5[183] | 2.0[183] | ng/mL | Narrow therapeutic window |
| 0.6[183] | 2.6[183] | nmol/L | ||
| Lithium | 0.4,[184] 0.5,[185][186] 0.8[187] | 1.3[185][186] | mmol/L | Narrow therapeutic window |
| Paracetamol | 30[188] | mg/L | Risk of paracetamol toxicity at higher levels | |
| 200[188] | µmol/L |
See also
- Cardiology diagnostic tests and procedures
- Comprehensive metabolic panel
- Medical technologist
- Reference range
Notes
- ↑ The MCHC in g/dL and the mass fraction of hemoglobin in red blood cells in % are numerically identical in practice, assuming a RBC density of 1g/mL and negligible hemoglobin in plasma.
References
- ↑ "Reference Ranges and What They Mean". Lab Tests Online (USA). http://labtestsonline.org/understanding/features/ref-ranges/start/6.
- ↑ Page 19 in: Stephen K. Bangert MA MB BChir MSc MBA FRCPath; William J. Marshall MA MSc MBBS FRCP FRCPath FRCPEdin FIBiol; Marshall, William Leonard (2008). Clinical biochemistry: metabolic and clinical aspects. Philadelphia: Churchill Livingstone/Elsevier. ISBN 978-0-443-10186-1.
- ↑ Bransky A, Larsson A, Aardal E, Ben-Yosef Y, Christenson RH (2021). "A Novel Approach to Hematology Testing at the Point of Care.". J Appl Lab Med 6 (2): 532–542. doi:10.1093/jalm/jfaa186. PMID 33274357.
- ↑ "Units of measurement" in Medical toxicology, Richard C. Dart Edition: 3, illustrated, Lippincott Williams & Wilkins, 2004, p. 34 ISBN:978-0-7817-2845-4 1914 pages
- ↑ 5.000 5.001 5.002 5.003 5.004 5.005 5.006 5.007 5.008 5.009 5.010 5.011 5.012 5.013 5.014 5.015 5.016 5.017 5.018 5.019 5.020 5.021 5.022 5.023 5.024 5.025 5.026 5.027 5.028 5.029 5.030 5.031 5.032 5.033 5.034 5.035 5.036 5.037 5.038 5.039 5.040 5.041 5.042 5.043 5.044 5.045 5.046 5.047 5.048 5.049 5.050 5.051 5.052 5.053 5.054 5.055 5.056 5.057 5.058 5.059 5.060 5.061 5.062 5.063 5.064 5.065 5.066 5.067 5.068 5.069 5.070 5.071 5.072 5.073 5.074 5.075 5.076 5.077 5.078 5.079 5.080 5.081 5.082 5.083 5.084 5.085 5.086 5.087 5.088 5.089 5.090 5.091 5.092 5.093 5.094 5.095 5.096 5.097 5.098 5.099 5.100 5.101 5.102 5.103 5.104 5.105 5.106 5.107 5.108 5.109 5.110 5.111 5.112 5.113 5.114 5.115 5.116 5.117 5.118 5.119 5.120 5.121 5.122 5.123 5.124 5.125 5.126 Reference range list from Uppsala University Hospital ("Laborationslista"). Artnr 40284 Sj74a. Issued on April 22, 2008
- ↑ 6.0 6.1 6.2 "Arterial versus venous reference ranges", Medical Laboratory Observer, April, 2000 by D. Robert Dufour
- ↑ 7.0 7.1 7.2 7.3 7.4 Ashvarya Mangla. "Troponins". http://emedicine.medscape.com/article/2073935-overview. Updated: Jan 14, 2015
- ↑ 8.0 8.1 8.2 8.3 "Gray zone BNP levels in heart failure patients in the emergency department: results from the Rapid Emergency Department Heart Failure Outpatient Trial (REDHOT) multicenter study". American Heart Journal 151 (5): 1006–11. May 2006. doi:10.1016/j.ahj.2005.10.017. PMID 16644322.
- ↑ 9.0 9.1 9.2 9.3 "Impact of the history of congestive heart failure on the utility of B-type natriuretic peptide in the emergency diagnosis of heart failure: results from the Breathing Not Properly Multinational Study". The American Journal of Medicine 119 (1): 69.e1–11. January 2006. doi:10.1016/j.amjmed.2005.04.029. PMID 16431187.
- ↑ Abbassi-Ghanavati, M.; Greer, L. G.; Cunningham, F. G. (2009). "Pregnancy and Laboratory Studies". Obstetrics & Gynecology 114 (6): 1326–31. doi:10.1097/AOG.0b013e3181c2bde8. PMID 19935037.
- ↑ Armbruster, David; Miller (August 2007). "The Joint Committee for Traceability in Laboratory Medicine (JCTLM): A Global Approach to Promote the Standardisation of Clinical Laboratory Test Results". The Clinical Biochemist Reviews 28 (3): 105–14. PMID 17909615.
- ↑ William Q. Meeker & Gerald J. Hahn (1982). "Sample Sizes for Prediction Intervals". Journal of Quality Technology 14 (4): 201–206. doi:10.1080/00224065.1982.11978821.
- ↑ PROOPIOMELANOCORTIN; NCBI --> POMC Retrieved on September 28, 2009
- ↑ 14.000 14.001 14.002 14.003 14.004 14.005 14.006 14.007 14.008 14.009 14.010 14.011 14.012 14.013 14.014 14.015 14.016 14.017 14.018 14.019 14.020 14.021 14.022 14.023 14.024 14.025 14.026 14.027 14.028 14.029 14.030 14.031 14.032 14.033 14.034 14.035 14.036 14.037 14.038 14.039 14.040 14.041 14.042 14.043 14.044 14.045 14.046 14.047 14.048 14.049 14.050 14.051 14.052 14.053 14.054 14.055 14.056 14.057 14.058 14.059 14.060 14.061 14.062 14.063 14.064 14.065 14.066 14.067 14.068 14.069 14.070 14.071 14.072 14.073 14.074 14.075 14.076 14.077 14.078 14.079 14.080 14.081 14.082 14.083 14.084 14.085 14.086 14.087 14.088 14.089 14.090 14.091 14.092 14.093 14.094 14.095 14.096 14.097 14.098 14.099 14.100 14.101 14.102 14.103 14.104 14.105 14.106 14.107 Last page of Deepak A. Rao; Le, Tao; Bhushan, Vikas (2007). First Aid for the USMLE Step 1 2008 (First Aid for the Usmle Step 1). McGraw-Hill Medical. ISBN 978-0-07-149868-5. https://archive.org/details/firstaidforusmle00taol.
- ↑ 15.000 15.001 15.002 15.003 15.004 15.005 15.006 15.007 15.008 15.009 15.010 15.011 15.012 15.013 15.014 15.015 15.016 15.017 15.018 15.019 15.020 15.021 15.022 15.023 15.024 15.025 15.026 15.027 15.028 15.029 15.030 15.031 15.032 15.033 15.034 15.035 15.036 15.037 15.038 15.039 15.040 15.041 15.042 15.043 15.044 15.045 15.046 15.047 15.048 15.049 15.050 15.051 15.052 15.053 15.054 15.055 15.056 15.057 15.058 15.059 15.060 15.061 15.062 15.063 15.064 15.065 15.066 15.067 15.068 15.069 15.070 15.071 15.072 15.073 15.074 15.075 15.076 15.077 15.078 15.079 15.080 15.081 15.082 15.083 15.084 15.085 15.086 15.087 15.088 15.089 15.090 15.091 15.092 15.093 15.094 15.095 15.096 15.097 15.098 15.099 15.100 15.101 15.102 15.103 15.104 15.105 Normal Reference Range Table from The University of Texas Southwestern Medical Center at Dallas. Used in Interactive Case Study Companion to Pathologic basis of disease.
- ↑ 16.0 16.1 16.2 16.3 Derived from molar values using molar mass of 22.99 g•mol−1
- ↑ 17.0 17.1 Derived from molar values using molar mass of 39.10 g•mol−1
- ↑ 18.00 18.01 18.02 18.03 18.04 18.05 18.06 18.07 18.08 18.09 18.10 18.11 18.12 Merck Manuals > Common Medical Tests > Blood Tests Last full review/revision February 2003
- ↑ 19.0 19.1 Derived from molar values using molar mass of 35.45 g•mol−1
- ↑ 20.0 20.1 "Serum ionized calcium and corrected total calcium in borderline hyperparathyroidism". Clin. Chem. 24 (11): 1962–65. November 1978. doi:10.1093/clinchem/24.11.1962. PMID 709830. http://www.clinchem.org/cgi/pmidlookup?view=long&pmid=709830.
- ↑ 21.0 21.1 21.2 21.3 Derived from molar values using molar mass of 40.08 g•mol−1
- ↑ 22.0 22.1 22.2 Derived from mass values using molar mass of 40.08 g•mol−1
- ↑ 23.00 23.01 23.02 23.03 23.04 23.05 23.06 23.07 23.08 23.09 23.10 23.11 23.12 23.13 23.14 23.15 23.16 23.17 23.18 23.19 23.20 23.21 23.22 23.23 23.24 23.25 23.26 23.27 23.28 23.29 23.30 23.31 23.32 23.33 23.34 23.35 23.36 23.37 23.38 23.39 23.40 23.41 23.42 23.43 23.44 23.45 23.46 23.47 23.48 23.49 23.50 23.51 23.52 23.53 23.54 23.55 23.56 23.57 23.58 23.59 23.60 23.61 23.62 23.63 23.64 23.65 23.66 23.67 23.68 23.69 23.70 23.71 23.72 23.73 23.74 23.75 23.76 23.77 Blood Test Results – Normal Ranges Bloodbook.Com
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 24.10 24.11 Slon S (2006-09-22). "Serum Iron". University of Illinois Medical Center. http://uimc.discoveryhospital.com/main.php?t=enc&id=1456.
- ↑ 25.0 25.1 25.2 25.3 Diagnostic Chemicals Limited > Serum Iron-SL Assay July 15, 2005
- ↑ 26.00 26.01 26.02 26.03 26.04 26.05 26.06 26.07 26.08 26.09 26.10 26.11 26.12 Derived from mass values using molar mass of 55.85 g•mol−1
- ↑ 27.0 27.1 Table 1. Page 133" Clinical Chemistry 45, No. 1, 1999 (stating 1.9–3.3 g/L)
- ↑ 28.0 28.1 Derived by dividing mass values with molar mass
- ↑ 29.0 29.1 29.2 29.3 Ferritin by: Mark Levin, MD, Hematologist and Oncologist, Newark, NJ. Review provided by VeriMed Healthcare Network
- ↑ 30.0 30.1 Andrea Duchini. "Hemochromatosis Workup". http://emedicine.medscape.com/article/177216-workup#c8. Updated: Jan 02, 2016
- ↑ 31.0 31.1 31.2 31.3 31.4 Derived from mass values using molar mass of 450,000 g•mol−1
- ↑ 32.0 32.1 "Metastatic carcinomatous cirrhosis and hepatic hemosiderosis in a patient heterozygous for the H63D genotype". Arch. Pathol. Lab. Med. 125 (8): 1084–87. August 2001. doi:10.5858/2001-125-1084-MCCAHH. PMID 11473464. http://journals.allenpress.com/jrnlserv/?request=get-abstract&issn=0003-9985&volume=125&page=1084.
- ↑ 33.0 33.1 "Reference intervals for blood ammonia in healthy subjects, determined by microdiffusion". Clin. Chem. 41 (7): 1048. July 1995. doi:10.1093/clinchem/41.7.1048a. PMID 7600690.
- ↑ 34.0 34.1 34.2 34.3 Derived from molar values using molar mass of 17.03 g/mol
- ↑ 35.0 35.1 Derived from mass values using molar mass of 63.55 g•mol−1
- ↑ "Reference range for copper". GPnotebook. https://www.gpnotebook.co.uk/simplepage.cfm?ID=1040580630.
- ↑ 37.0 37.1 Derived from mass using molar mass of 151kDa
- ↑ Walter F. Boron (2005). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 849. ISBN 978-1-4160-2328-9.
- ↑ 39.0 39.1 "Archived copy". http://www.dlolab.com/PDFs/DLO-OCTOBER-2008-LAB-UPDATE.pdf.
- ↑ 40.0 40.1 Derived from molar values using molar mass of 65.38 g/mol
- ↑ 41.0 41.1 Derived from mass values using molar mass of 65.38 g/mol
- ↑ 42.0 42.1 Derived from molar values using molar mass of 24.31 g/mol
- ↑ 43.0 43.1 Derived from mass values using molar mass of 24.31 g/mol
- ↑ "Agreements between arterial and central venous values for pH, bicarbonate, base excess, and lactate". Emerg Med J 23 (8): 622–24. August 2006. doi:10.1136/emj.2006.035915. PMID 16858095.
- ↑ 45.00 45.01 45.02 45.03 45.04 45.05 45.06 45.07 45.08 45.09 45.10 45.11 The Medical Education Division of the Brookside Associates--> ABG (Arterial Blood Gas) Retrieved on Dec 6, 2009
- ↑ 46.0 46.1 Derived from molar values using molar mass of 1.01 g•mol−1
- ↑ 47.0 47.1 47.2 47.3 47.4 47.5 47.6 47.7 Derived from mmHg values using 0.133322 kPa/mmHg
- ↑ 48.0 48.1 Derived from molar values using molar mass of 44.010 g/mol
- ↑ 49.0 49.1 49.2 49.3 Derived from molar values using molar mass of 61 g/mol
- ↑ "Reference range (albumin)". GPnotebook. https://www.gpnotebook.co.uk/simplepage.cfm?ID=288686147.
- ↑ 51.0 51.1 Derived from mass using molecular weight of 65kD
- ↑ 52.0 52.1 52.2 52.3 52.4 Derived from mass values using molar mass of 585g/mol
- ↑ 53.0 53.1 Derived from molar values using molar mass of 585g/mol
- ↑ 54.00 54.01 54.02 54.03 54.04 54.05 54.06 54.07 54.08 54.09 54.10 54.11 54.12 54.13 54.14 54.15 54.16 54.17 54.18 Fachwörterbuch Kompakt Medizin E-D/D-E. Author: Fritz-Jürgen Nöhring. Edition 2. Publisher:Elsevier, Urban&FischerVerlag, 2004. ISBN:978-3-437-15120-0. Length: 1288 pages
- ↑ 55.0 55.1 55.2 55.3 GPnotebook > reference range (AST) Retrieved on Dec 7, 2009
- ↑ 56.0 56.1 "Gamma-GT". Leistungsverzeichnis. Medizinisch-Diagnostische Institute. http://www.mdi-labor.de/l_leistungsverzeichnis_detail.php?u_id=663&init=letter.
- ↑ 57.0 57.1 "Creatine kinase". GPnotebook. https://www.gpnotebook.co.uk/simplepage.cfm?ID=1436155929.
- ↑ 58.0 58.1 58.2 58.3 Page 585 in: Lee, Mary Ann (2009). Basic Skills in Interpreting Laboratory Data. Amer Soc of Health System. ISBN 978-1-58528-180-0.
- ↑ 59.0 59.1 59.2 59.3 Muscle Information and Courses from MediaLab, Inc. > Cardiac Biomarkers Retrieved on April 22, 2010
- ↑ Caselli, C.; Cangemi, G.; Masotti, S.; Ragusa, R.; Gennai, I.; Del Ry, S.; Prontera, C.; Clerico, A. (2016-07-01). "Plasma cardiac troponin I concentrations in healthy neonates, children and adolescents measured with a high sensitive immunoassay method: High sensitive troponin I in pediatric age" (in en). Clinica Chimica Acta 458: 68–71. doi:10.1016/j.cca.2016.04.029. ISSN 0009-8981. PMID 27118089. https://www.sciencedirect.com/science/article/abs/pii/S0009898116301553.
- ↑ Baum, Hannsjörg; Hinze, Anika; Bartels, Peter; Neumeier, Dieter (2004-12-01). "Reference values for cardiac troponins T and I in healthy neonates" (in en). Clinical Biochemistry 37 (12): 1079–82. doi:10.1016/j.clinbiochem.2004.08.003. ISSN 0009-9120. PMID 15589813. https://www.sciencedirect.com/science/article/pii/S0009912004002218.
- ↑ 62.0 62.1 Page 220 in: Lee, Mary Ann (2009). Basic Skills in Interpreting Laboratory Data. Amer Soc of Health System. ISBN 978-1-58528-180-0.
- ↑ 63.00 63.01 63.02 63.03 63.04 63.05 63.06 63.07 63.08 63.09 63.10 63.11 63.12 63.13 Adëeva Nutritionals Canada > Optimal blood test values Retrieved on July 9, 2009
- ↑ 64.0 64.1 64.2 64.3 64.4 64.5 Derived from values in mg/dL to mmol/L, by dividing by 89, according to faqs.org: What are mg/dL and mmol/L? How to convert? Glucose? Cholesterol? Last Update July 21, 2009. Retrieved on July 21, 2009
- ↑ 65.0 65.1 65.2 65.3 Derived from values in mg/dL to mmol/L, using molar mass of 386.65 g/mol
- ↑ 66.0 66.1 66.2 "Reference range (cholesterol)". GPnotebook. https://www.gpnotebook.co.uk/simplepage.cfm?ID=-214630397.
- ↑ 67.0 67.1 67.2 67.3 67.4 67.5 67.6 67.7 Royal College of Pathologists of Australasia; Cholesterol (HDL and LDL) – plasma or serum Last Updated: Monday, 6 August 2007
- ↑ 68.0 68.1 68.2 68.3 68.4 68.5 68.6 68.7 68.8 68.9 Derived from values in mmol/L, using molar mass of 386.65 g/mol
- ↑ What Your Cholesterol Levels Mean. American Heart Association. Retrieved on September 12, 2009
- ↑ "HDL Cholesterol: The Test". September 3, 2001. http://www.labtestsonline.org/understanding/analytes/hdl/test.html.
- ↑ GP Notebook > range (reference, ca-125) Retrieved on Jan 5, 2009
- ↑ ClinLab Navigator > Test Interpretations > CA-125 Retrieved on March 8, 2011
- ↑ 73.0 73.1 "Reference intervals for carcinoembryonic antigen (CEA), CA125, MUC1, Alfa-foeto-protein (AFP), neuron-specific enolase (NSE) and CA19.9 from the NORIP study". Scandinavian Journal of Clinical and Laboratory Investigation 68 (8): 703–13. June 2008. doi:10.1080/00365510802126836. PMID 18609108. https://figshare.com/articles/journal_contribution/11808120.
- ↑ Carcinoembryonic Antigen(CEA) at MedicineNet
- ↑ 75.0 75.1 75.2 Luboldt, Hans-Joachim; Schindler, Joachim F.; Rübben, Herbert (2007). "Age-Specific Reference Ranges for Prostate-Specific Antigen as a Marker for Prostate Cancer". EAU-EBU Update Series 5 (1): 38–48. doi:10.1016/j.eeus.2006.10.003. ISSN 1871-2592.
- ↑ 76.0 76.1 76.2 "Reference intervals for serum calcitonin in men, women, and children". Clinical Chemistry 50 (10): 1828–30. October 2004. doi:10.1373/clinchem.2003.026963. PMID 15388660.
- ↑ The TSH Reference Range Wars: What's "Normal?", Who is Wrong, Who is Right... By Mary Shomon, About.com. Updated: June 19, 2006. About.com Health's Disease and Condition
- ↑ 78.0 78.1 2006 Press releases: Thyroid Imbalance? Target Your Numbers Contacts: Bryan Campbell American] Association of Clinical Endocrinologists
- ↑ 79.0 79.1 The TSH Reference Range Wars: What's "Normal?", Who is Wrong, Who is Right... By Mary Shomon, About.com. Updated: June 19, 2006
- ↑ 80.0 80.1 Demers, Laurence M.; Carole A. Spencer (2002). "LMPG: Laboratory Support for the Diagnosis and Monitoring of Thyroid Disease". National Academy of Clinical Biochemistry (USA). http://www.nacb.org/lmpg/thyroid_LMPG_PDF.stm. – see Section 2. Pre-analytic factors
- ↑ 81.0 81.1 81.2 81.3 81.4 81.5 Free T4; Thyroxine, Free; T4, Free UNC Health Care System
- ↑ Derived from molar values using molar mass of 776.87 g/mol
- ↑ 83.0 83.1 83.2 83.3 83.4 83.5 Derived from mass values using molar mass of 776.87 g/mol
- ↑ 84.00 84.01 84.02 84.03 84.04 84.05 84.06 84.07 84.08 84.09 84.10 84.11 84.12 84.13 84.14 Table 4: Typical reference ranges for serum assays – Thyroid Disease Manager
- ↑ 85.0 85.1 85.2 85.3 "Euthyroid patient with elevated serum free thyroxine". Clinical Chemistry 54 (7): 1239–41. July 2008. doi:10.1373/clinchem.2007.101428. PMID 18593963.
- ↑ 86.0 86.1 86.2 86.3 Derived from mass values using molar mass of 650.98 g/mol
- ↑ 87.0 87.1 "Serum concentration of free T3, free T4 and TSH in healthy children". Journal of Pediatric Endocrinology & Metabolism 14 (9): 1635–39. 2001. doi:10.1515/jpem.2001.14.9.1635. INIST:13391788. PMID 11795654.
- ↑ Häggström, Mikael (2014). "Reference ranges for estradiol, progesterone, luteinizing hormone and follicle-stimulating hormone during the menstrual cycle". WikiJournal of Medicine 1 (1). doi:10.15347/wjm/2014.001.
- ↑ 89.0 89.1 "Andrology Australia: Your Health > Low Testosterone > Diagnosis". http://www.andrologyaustralia.org/pageContent.asp?pageCode=LOWTESTDIAG#LOWTESTDIAGNORM.
- ↑ 90.0 90.1 90.2 90.3 Derived from mass values using molar mass of 288.42g/mol
- ↑ 91.0 91.1 91.2 91.3 91.4 91.5 91.6 Derived from molar values using molar mass of 288.42g/mol
- ↑ 92.0 92.1 92.2 92.3 MedlinePlus > Testosterone Update Date: 3/18/2008. Updated by: Elizabeth H. Holt, MD, PhD, Yale University. Review provided by VeriMed Healthcare Network. Also reviewed by David Zieve, MD, MHA, Medical Director
- ↑ 93.0 93.1 93.2 93.3 Derived from mass values using molar mass of 330.46g/mol
- ↑ 94.0 94.1 94.2 94.3 94.4 94.5 94.6 94.7 94.8 94.9 reference range (FSH) GPnotebook. Retrieved on September 27, 2009
- ↑ 95.0 95.1 95.2 95.3 95.4 95.5 Values taken from day 1 after LH surge in: "Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer". Clinical Chemistry and Laboratory Medicine 44 (7): 883–87. 2006. doi:10.1515/CCLM.2006.160. PMID 16776638.
- ↑ 96.0 96.1 96.2 96.3 96.4 96.5 New York Hospital Queens > Services and Facilities > Patient Testing > Pathology > New York Hospital Queens Diagnostic Laboratories > Test Directory > Reference Ranges[yes|permanent dead link|dead link}}] Retrieved on Nov 8, 2009
- ↑ 97.0 97.1 Mayo Medical Laboratories > Test ID: LH, Luteinizing Hormone (LH), Serum , retrieved December 2012
- ↑ 98.0 98.1 98.2 98.3 98.4 98.5 98.6 GPNotebook – reference range (oestradiol) Retrieved on September 27, 2009
- ↑ 99.0 99.1 99.2 99.3 99.4 99.5 99.6 Derived from molar values using molar mass of 272.38g/mol
- ↑ 100.0 100.1 100.2 100.3 Total amount multiplied by 0.022 according to 2.2% presented in: "Free and protein-bound plasma estradiol-17 beta during the menstrual cycle". J. Clin. Endocrinol. Metab. 43 (2): 436–45. August 1976. doi:10.1210/jcem-43-2-436. PMID 950372.
- ↑ 101.0 101.1 Derived from mass values using molar mass of 314.46 g/mol
- ↑ 102.0 102.1 Bhattacharya Sudhindra Mohan (July/August 2005) Mid-luteal phase plasma progesterone levels in spontaneous and clomiphene citrate induced conception cycles J Obstet Gynecol India Vol. 55, No. 4 : July/August 2005 pp. 350–52
- ↑ 103.0 103.1 Dehydroepiandrosterone Sulfate (DHEA-S), Serum at Mayo Foundation For Medical Education And Research. Retrieved July 2012
- ↑ 104.0 104.1 104.2 104.3 Unit Code 91215 at Mayo Clinic Medical Laboratories. Retrieved April 2011
- ↑ 105.0 105.1 Antimullerian Hormone (AMH), Serum from Mayo Medical Laboratories. Retrieved April 2012.
- ↑ 106.0 106.1 Derived from mass values using 140,000 g/mol, as given in:
- "Antimüllerian hormone (AMH) not only a marker for prediction of ovarian reserve". Physiological Research 60 (2): 217–23. 2011. doi:10.33549/physiolres.932076. PMID 21114374.
- ↑ 107.0 107.1 Nieman, Lynnette K (29 September 2019). "Measurement of ACTH, CRH, and other hypothalamic and pituitary peptides". UpToDate. https://www.uptodate.com/contents/measurement-of-acth-crh-and-other-hypothalamic-and-pituitary-peptides.
- ↑ 108.0 108.1 108.2 108.3 Biochemistry Reference Ranges at Good Hope Hospital Retrieved on Nov 8, 2009
- ↑ 109.0 109.1 109.2 109.3 Derived from molar values using molar mass of 362 g/mol
- ↑ 110.0 110.1 110.2 110.3 110.4 110.5 110.6 110.7 "Reference ranges of serum IGF-1 and IGFBP-3 levels in a general adult population: results of the Study of Health in Pomerania (SHIP)". Growth Hormone & IGF Research 18 (3): 228–37. June 2008. doi:10.1016/j.ghir.2007.09.005. PMID 17997337.
- ↑ 111.00 111.01 111.02 111.03 111.04 111.05 111.06 111.07 111.08 111.09 111.10 111.11 111.12 111.13 111.14 111.15 Taken from the assay method giving the lowest and highest estimate, respectively, from Table 2 in: "Serum total prolactin and monomeric prolactin reference intervals determined by precipitation with polyethylene glycol: evaluation and validation on common immunoassay platforms". Clinical Chemistry 54 (10): 1673–81. October 2008. doi:10.1373/clinchem.2008.105312. PMID 18719199.
- ↑ 112.0 112.1 Derived from molar values using molar mass of 9.4 kDa
- ↑ 113.0 113.1 Table 2 in: "Reference range for serum parathyroid hormone". Endocr Pract 12 (2): 137–44. 2006. doi:10.4158/ep.12.2.137. PMID 16690460.
- ↑ 114.0 114.1 Derived from mass values using molar mass of 9.4 kDa
- ↑ 115.0 115.1 115.2 115.3 115.4 115.5 Derived from molar values using molar mass 400.6 g/mol
- ↑ 116.0 116.1 116.2 116.3 Bender, David A. (2003). "Vitamin D". Nutritional biochemistry of the vitamins. Cambridge: Cambridge University Press. ISBN 978-0-521-80388-5. https://books.google.com/books?id=pxEJNs0IUo4C. Retrieved December 10, 2008 through Google Book Search.
- ↑ 117.0 117.1 117.2 117.3 "Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y". The American Journal of Clinical Nutrition 80 (3): 752–58. September 2004. doi:10.1093/ajcn/80.3.752. PMID 15321818.
- ↑ 118.0 118.1 118.2 118.3 "Cyclic changes of vitamin D and PTH are primarily regulated by solar radiation: 5-year analysis of a German (50 degrees N) population". Horm. Metab. Res. 41 (5): 402–07. May 2009. doi:10.1055/s-0028-1128131. PMID 19241329.
- ↑ 119.0 119.1 119.2 119.3 119.4 119.5 119.6 119.7 "Calcium and vitamin D in preventing fractures: data are not sufficient to show inefficacy". BMJ 331 (7508): 108–09; author reply 109. July 2005. doi:10.1136/bmj.331.7508.108-b. PMID 16002891.
- ↑ 120.0 120.1 Converted from values in mcU/mL by dividing with a factor of 11.2 mcU/mL per ng/(mL*hour), as given in: Washington, Department of Laboratory Medicine. Retrieved Mars 2011
- ↑ 121.0 121.1 "Different secretory pathways of renin from mouse cells transfected with the human renin gene". The Journal of Biological Chemistry 263 (7): 3137–41. March 1988. doi:10.1016/S0021-9258(18)69046-5. PMID 2893797.
- ↑ 122.0 122.1 122.2 122.3 New Assays for Aldosterone, Renin and Parathyroid Hormone University of Washington, Department of Laboratory Medicine. Retrieved Mars 2011
- ↑ 123.0 123.1 Converted from values in ng/(mL*hour) by multiplying with a factor of 11.2 mcU/mL per ng/(mL*hour), as given in: Washington, Department of Laboratory Medicine. Retrieved Mars 2011
- ↑ 124.0 124.1 Converted from mass values using molar mass of 360.44 g/mol
- ↑ 125.0 125.1 125.2 125.3 "The use of aldosterone-renin ratio as a diagnostic test for primary hyperaldosteronism and its test characteristics under different conditions of blood sampling". The Journal of Clinical Endocrinology and Metabolism 90 (1): 72–78. January 2005. doi:10.1210/jc.2004-1149. PMID 15483077.
- ↑ 126.0 126.1 126.2 126.3 126.4 126.5 Central Manchester University Hospitals --> Reference ranges Retrieved on July 9, 2009
- ↑ University of Kentucky Chandler Medical Center > Clinical Lab Reference Range Guide Retrieved on April 28, 2009
- ↑ 128.0 128.1 128.2 128.3 128.4 Derived from mass values using molar mass of 441 mol−1
- ↑ 129.0 129.1 GPnotebook > B12 Retrieved on April 28, 2009
- ↑ 130.0 130.1 Derived form molar values using molar mass of 1355g/mol
- ↑ 131.0 131.1 Derived from mass values using molar mass of 1355g/mol
- ↑ 132.0 132.1 132.2 132.3 "Homocysteine". http://www.thedoctorsdoctor.com/labtests/homocysteine.htm.
- ↑ 133.0 133.1 133.2 133.3 Derived from molar values using molar massof 135 g/mol
- ↑ 134.0 134.1 Derived from mass values using molar mass of 176 grams per mol
- ↑ 135.0 135.1 135.2 For Driving under the influence by country, see Drunk driving law by country
- ↑ Derived from mass values using molar mass of 46g/mol
- ↑ 137.0 137.1 137.2 137.3 137.4 Derived from mass values using 64,500 g/mol. This molar mass was taken from: "Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle". J Appl Physiol 90 (2): 511–19. 2001. doi:10.1152/jappl.2001.90.2.511. PMID 11160049.
- ↑ 138.0 138.1 138.2 138.3 Normal Lab Values at Marshall University Joan C. Edwards School of Medicine. Retrieved July 2013
- ↑ 139.0 139.1 139.2 139.3 139.4 139.5 139.6 139.7 molar concentration as given for hemoglobin above, but multiplied by 4, according to: "Wrong molar hemoglobin reference values-a longstanding error that should be corrected". Annals of Hematology 89 (2): 209. February 2010. doi:10.1007/s00277-009-0791-x. PMID 19609525.
- ↑ 140.0 140.1 140.2 140.3 Derived from mass concentration, using molar mass of 64,458 g/mol. This molar mass was taken from: "Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle". J Appl Physiol 90 (2): 511–19. 2001. doi:10.1152/jappl.2001.90.2.511. PMID 11160049. . Subsequently, 1 g/dL = 0.1551 mmol/L
- ↑ 141.0 141.1 141.2 141.3 141.4 141.5 Morkis IV, Farias MG, Scotti L (2016). "Determination of reference ranges for immature platelet and reticulocyte fractions and reticulocyte hemoglobin equivalent.". Rev Bras Hematol Hemoter 38 (4): 310–313. doi:10.1016/j.bjhh.2016.07.001. PMID 27863758.
- ↑ 142.0 142.1 Brugnara C, Schiller B, Moran J (2006). "Reticulocyte hemoglobin equivalent (Ret He) and assessment of iron-deficient states.". Clinical and Laboratory Haematology 28 (5): 303–8. doi:10.1111/j.1365-2257.2006.00812.x. PMID 16999719.
- ↑ 143.0 143.1 143.2 143.3 143.4 143.5 143.6 143.7 lymphomation.org > Tests & Imaging > Labs > Complete Blood Count Retrieved on May 14, 2009
- ↑ 144.00 144.01 144.02 144.03 144.04 144.05 144.06 144.07 144.08 144.09 144.10 144.11 144.12 144.13 144.14 144.15 144.16 144.17 144.18 144.19 144.20 McClatchey, Kenneth D. (November 28, 2002). Clinical Laboratory Medicine. Lippincott Williams & Wilkins. ISBN 9780683307511. https://books.google.com/books?id=3PJVLH1NmQAC.
- ↑ "Determination of monocyte count by hematological analyzers, manual method and flow cytometry in Polish population" Central European Journal of Immunology (Centr Eur J Immunol 2006; 31 (1–2): 1–5) authors: Elżbieta Górska, Urszula Demkow, Roman Pińkowski, Barbara Jakubczak, Dorota Matuszewicz, Jolanta Gawęda, Wioletta Rzeszotarska, Maria Wąsik,
- ↑ 146.0 146.1 146.2 146.3 146.4 gpnotebook.co.uk > blood constituents (reference range) Retrieved on May 14, 2009
- ↑ 147.0 147.1 "Normal range of mean platelet volume in healthy subjects: Insight from a large epidemiologic study". Thromb. Res. 128 (4): 358–60. 2011. doi:10.1016/j.thromres.2011.05.007. PMID 21620440.
- ↑ 148.0 148.1 Normal Values: RBC, Hgb, Hct, Indices, RDW, Platelets, and MPV (Conventional Units) From labcareplus. Retrieved 4 nov, 2010
- ↑ 149.0 149.1 "[Platelet count and mean platelet volume in the Spanish population]" (in es). Med Clin (Barc) 110 (20): 774–77. 1998. PMID 9666418.
- ↑ 150.0 150.1 MedlinePlus Encyclopedia 003652
- ↑ 151.0 151.1 Antithrombin III at eMedicine
- ↑ 152.0 152.1 Antithrombin CO000300 in Coagulation Test Handbook at Massachusetts General Hospital. In turn citing:
- Elizabeth M. Van Cott, M.D., and Michael Laposata, M.D., Ph.D., "Coagulation." In: Jacobs DS et al, ed. The Laboratory Test Handbook, 5th Edition. Lexi-Comp, Cleveland, 2001; 327–58.
- ↑ 153.0 153.1 "Home". http://pathology.bsuh.nhs.uk/pathology/Default.aspx?tabid=108.
- ↑ 154.0 154.1 "Simple rule for calculating normal erythrocyte sedimentation rate". British Medical Journal 286 (6361): 266. January 1983. doi:10.1136/bmj.286.6361.266. PMID 6402065.
- ↑ "Normal erythrocyte sedimentation rate and age". Br Med J 2 (5544): 85–87. 1967. doi:10.1136/bmj.2.5544.85. PMID 6020854.
- ↑ "C-reactive protein". GPnotebook. https://www.gpnotebook.co.uk/simplepage.cfm?ID=946536472.
- ↑ 2730 Serum C-Reactive Protein values in Diabetics with Periodontal Disease A.R. Choudhury, and S. Rahman, Birdem, Diabetic Association of Bangladesh, Dhaka, Bangladesh. (the diabetics were not used to determine the reference ranges)
- ↑ 158.0 158.1 158.2 158.3 Derived from mass using molar mass of 25,106 g/mol
- ↑ 159.0 159.1 "Alpha-1 antitrypsin deficiency: an overlooked cause of late hemorrhagic disease of the newborn". Journal of Pediatric Hematology/Oncology 25 (3): 274–75. March 2003. doi:10.1097/00043426-200303000-00019. PMID 12621252.
- ↑ 160.0 160.1 Derived from mass values using molar mass of 44324.5 g/mol
- ↑ 161.0 161.1 Derived from molar values using molar mass of 44324.5 g/mol
- ↑ "Procalcitonin, Serum". Mayo Clinic. http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/83169.
- ↑ 163.0 163.1 163.2 163.3 163.4 163.5 163.6 163.7 163.8 163.9 The Society for American Clinical Laboratory Science > Chemistry Tests > Immunoglobulins Retrieved on Nov 26, 2009
- ↑ 164.0 164.1 "SSA – Clinical: SS-A/Ro Antibodies, IgG, Serum". Mayo Clinic Laboratories. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/81360.
- ↑ 165.0 165.1 "SSB – Clinical: SS-B/La Antibodies, IgG, Serum". Mayo Clinic Laboratories. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/81359.
- ↑ 166.0 166.1 166.2 "ADNA – Clinical: DNA Double-Stranded Antibodies, IgG, Serum". Mayo Clinic Laboratories. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8178.
- ↑ 167.00 167.01 167.02 167.03 167.04 167.05 167.06 167.07 167.08 167.09 167.10 167.11 167.12 167.13 167.14 167.15 167.16 167.17 167.18 167.19 167.20 167.21 167.22 167.23 167.24 167.25 167.26 167.27 167.28 167.29 167.30 chronolab.com > Autoantibodies associated with rheumatic diseases > Reference ranges Retrieved on April 29, 2010
- ↑ 168.0 168.1 168.2 "AMA – Clinical: Mitochondrial Antibodies (M2), Serum". Mayo Clinic Laboratories. https://www.mayocliniclabs.com/test-catalog/Clinical+and+Interpretive/8176.
- ↑ 169.0 169.1 "Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance". Blood 106 (3): 812–17. August 2005. doi:10.1182/blood-2005-03-1038. PMID 15855274.
- ↑ "Reference range (amylase)". GPnotebook. https://www.gpnotebook.co.uk/simplepage.cfm?ID=309002307.
- ↑ "Plasma measurement of D-dimer levels for the early diagnosis of ischemic stroke subtypes". Archives of Internal Medicine 162 (22): 2589–93. 2002. doi:10.1001/archinte.162.22.2589. PMID 12456231.
- ↑ "D-dimer concentrations in normal pregnancy: new diagnostic thresholds are needed". Clinical Chemistry 51 (5): 825–29. May 2005. doi:10.1373/clinchem.2004.044883. PMID 15764641.
- ↑ 173.0 173.1 "Age- and sex-related reference ranges for eight plasma constituents derived from randomly selected adults in a Scottish new town". Journal of Clinical Pathology 33 (4): 380–85. April 1980. doi:10.1136/jcp.33.4.380. PMID 7400337.
- ↑ 174.0 174.1 174.2 174.3 "Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance". Annals of Clinical Biochemistry 37 (1): 49–59. January 2000. doi:10.1258/0004563001901524. PMID 10672373.
- ↑ 175.0 175.1 175.2 175.3 175.4 175.5 175.6 175.7 Derived from molar values by multiplying with the molar mass of 113.118 g/mol, and divided by 10.000 to adapt from μg/L to mg/dL
- ↑ 176.0 176.1 MedlinePlus Encyclopedia Glucose tolerance test
- ↑ 177.0 177.1 177.2 Derived from molar values using molar mass of 180g/mol
- ↑ 178.0 178.1 Derived from mass values using molar mass of 180g/mol
- ↑ 179.0 179.1 "Diabetes – Prevention". http://my.clevelandclinic.org/health/diseases_conditions/hic_Diabetes_Basics/hic_Understanding_Pre-Diabetes. Last revised 1/15/2013
- ↑ 180.0 180.1 180.2 180.3 Derived from mass values using molar mass of 90.08 g/mol
- ↑ 181.0 181.1 Derived from mass values using molar mass of 88.06 g/mol
- ↑ 182.0 182.1 Ketones at eMedicine
- ↑ 183.0 183.1 183.2 183.3 Page 700 in:
Richard C. Dart (2004). Medical Toxicology. Lippincott Williams & Wilkins=year=2004. ISBN 9780781728454. - ↑ The UK Electronic Medical Compendium recommends 0.4–0.8 mmol/L plasma lithium level in adults for prophylaxis of recurrent affective bipolar manic-depressive illness Camcolit 250 mg Lithium Carbonate Revision 2 December 2010, Retrieved 5 May 2011
- ↑ 185.0 185.1 Amdisen A. (1978). "Clinical and serum level monitoring in lithium therapy and lithium intoxication". J. Anal. Toxicol. 2 (5): 193–202. doi:10.1093/jat/2.5.193.
- ↑ 186.0 186.1 R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 851–54.
- ↑ One study (Solomon, D.; Ristow, W.; Keller, M.; Kane, J.; Gelenberg, A.; Rosenbaum, J.; Warshaw, M. (1996). "Serum lithium levels and psychosocial function in patients with bipolar I disorder". The American Journal of Psychiatry 153 (10): 1301–07. doi:10.1176/ajp.153.10.1301. PMID 8831438. ) concluded a "low" dose of 0.4–0.6 mmol/L serum lithium treatment for patients with bipolar 1 disorder had less side effects, but a higher rate of relapse, than a "standard" dose of 0.8–1.0 mmol/L. However, a reanalysis of the same experimental data (Perlis, R.; Sachs, G.; Lafer, B.; Otto, M.; Faraone, S.; Kane, J.; Rosenbaum, J. (2002). "Effect of abrupt change from standard to low serum levels of lithium: A reanalysis of double-blind lithium maintenance data". The American Journal of Psychiatry 159 (7): 1155–59. doi:10.1176/appi.ajp.159.7.1155. PMID 12091193. ) concluded the higher rate of relapse for the "low" dose was due to abrupt changes in the lithium serum levels[improper synthesis?]
- ↑ 188.0 188.1 John Marx; Ron Walls; Robert Hockberger (2013). Rosen's Emergency Medicine – Concepts and Clinical Practice. Elsevier Health Sciences. ISBN 9781455749874.
External links
Further reading
- Rappoport, n.; Paik, P.; Oskotsky, B.; Tor, R.; Ziv, E.; Zaitlen, N.; Butte, A. (4 November 2017). "Creating ethnicity-specific reference intervals for lab tests from EHR data". bioRxiv 10.1101/213892.
Common for blood tests (CPT 82000–84999) | |
|---|---|
| Electrolytes | |
| Acid-base | |
| Iron tests | |
| Hormones | |
| Metabolism | |
| Cardiovascular | |
| Liver function tests | |
| Pancreas | |
 | 0.00      (0 votes) (0 votes) |
Categories: [Blood tests]
↧ Download as ZWI file | Last modified: 02/09/2024 03:50:44 | 15 views
☰ Source: https://handwiki.org/wiki/Medicine:Reference_ranges_for_blood_tests | License: CC BY-SA 3.0
 KSF
KSF