Phenyl Isothiocyanate
 From Handwiki
From Handwiki
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Isothiocyanatobenzene[1] | |||
| Other names
Phenyl isothiocyanate[1]
Thiocarbanil | |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| ChemSpider |
| ||
PubChem CID
|
| ||
| UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula
|
C7H5NS | ||
| Molar mass | 135.19 g/mol | ||
| Appearance | Colorless liquid with a pungent odor[2] | ||
| Density | 1.1288 g/cm3[2] | ||
| Melting point | −21 °C (−6 °F; 252 K)[3] | ||
| Boiling point | 221 °C (430 °F; 494 K)[3] | ||
Solubility in water
|
negligible [2] | ||
| Solubility | ethanol, ether[3] | ||
Magnetic susceptibility (χ)
|
-86.0·10−6 cm3/mol | ||
| Hazards | |||
| Main hazards | toxic, flammable[2] | ||
| GHS pictograms |    [3] [3]
| ||
| GHS Signal word | Danger[3] | ||
GHS hazard statements
|
H331, H311, H301, H314, H317, H334, H361[3] | ||
GHS precautionary statements
|
P301+310, P280, P312, P302+350, P301+330+331, P305+351+338, P310, P261, P304+341, P342+311[3] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
- PairSizeSet
Phenyl isothiocyanate (PITC) is a reagent used in reversed phase HPLC. PITC is less sensitive than o-phthaldehyde (OPA) and cannot be fully automated. PITC can be used for analysing secondary amines, unlike OPA. It is also known as Edman's reagent and is used in Edman degradation.
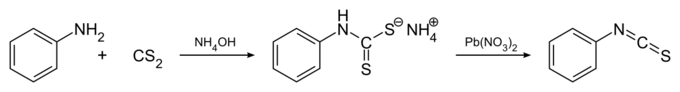
Commercially available, this compound may be synthesized by the reaction of aniline with carbon disulfide and concentrated ammonia to give the ammonium dithiocarbamate salt of aniline in the first step, which on further reaction with lead(II) nitrate gives phenyl isothiocyanate:[4]
Another method of synthesizing this reagent involves a Sandmeyer reaction using aniline, sodium nitrite and copper(I) thiocyanate.
A use of phenylisothiocyanate is in the synthesis of linogliride.[5]
See also
- Isothiocyanate
- Naphthyl isothiocyanate
References
- ↑ 1.0 1.1 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 665. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ 2.0 2.1 2.2 2.3 "Phenyl isothiocyanate - CAS # 103-72-0". http://www.caslab.com/Phenyl_isothiocyanate_CAS_103-72-0/.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Message". http://www.chemexper.com/cheminfo/servlet/org.dbcreator.MainServlet?query=entry._entryID=1288243&target=entry&action=PowerSearch&format=google2008.
- ↑ F. B. Dains, R. Q. Brewster, and C. P. Olander. "Phenyl isothiocyanate". Organic Syntheses. http://www.orgsyn.org/demo.aspx?prep=CV1P0447.; Collective Volume, 1, pp. 447
- ↑ U.S. Patent 4,211,867A
 |
Categories: [Isothiocyanates] [Foul-smelling chemicals] [Phenyl compounds]
↧ Download as ZWI file | Last modified: 08/23/2024 16:33:41 | 7 views
☰ Source: https://handwiki.org/wiki/Chemistry:Phenyl_isothiocyanate | License: CC BY-SA 3.0

 KSF
KSF