Dipole
 From Conservapedia
From Conservapedia 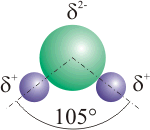
Dipole molecule
A dipole is a molecule which has two magnetic poles, caused by a non-zero total magnetic charge. The poles are normally denoted north and south, respectively. Common dipole molecules are water, ozone (but not atmospheric oxygen O2), and benzene.
As is well known, oil and water do not mix. This is because most or all oil molecules have a different molecular polarity than water.
See also[edit]
- Dipole antenna used in Amateur radio
Categories: [Chemistry] [Physics]
↧ Download as ZWI file | Last modified: 03/19/2023 08:50:00 | 8 views
☰ Source: https://www.conservapedia.com/Dipole | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: KSF
KSF