Silane
 From Nwe
From Nwe | Silane | |
|---|---|
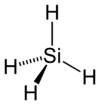  |
|
| General | |
| Systematic name | Silane |
| Other names | Silicon tetrahydride Silicon hydride Monosilane Silicane |
| Molecular formula | SiH4 |
| Molar mass | 32.12 g mol−1 |
| Appearance | Colourless gas |
| CAS number | [7803-62-5] |
| Properties | |
| Density and phase | ? kg m−3 (solid) 0.7 g/ml (liquid) 1.342 g L−1 (gas) |
| Solubility in water | Insoluble |
| Melting point | 88 K (−185°C) |
| Boiling point | 161 K (−112°C) |
| ΔfH0solid | -1615 kJ mol−1 |
| S0solid | 283 J mol−1 K−1 |
| Structure | |
| Molecular shape | tetrahedral |
| Dipole moment | 0 D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | low toxicity, avoid exposure to skin, irritant, may cause redness and swelling |
| NFPA 704 | |
| Flash point | N/A |
| Autoignition temperature | 294 K (21°C) |
| R/S statement | R: ? S: ? |
| UN number | 2203 |
| RTECS number | VV1400000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related silanes | disilane trisilane tetrasilane cyclosilane |
| Related hydrides | methane |
| Related compounds | disilene |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references |
|
Silane is a chemical compound with the chemical formula SiH4. It is the silicon analog of methane and, like methane, it is a gas at ordinary temperatures. The name "silane" is also given to a family of compounds that are silicon analogs of alkane hydrocarbons. Silanes consist of a chain of silicon atoms covalently bound to hydrogen atoms. The general formula of a silane is SinH2n+2.
Silanes are useful as coupling agents to bind glass fibers to polymers, and to couple a bio-inert layer on a titanium implant. They are also used for water repellents, sealants, masonry protection, graffiti control, semiconductor manufacturing processes, and chemical reduction reactions.
Nomenclature of different structures
There are certain rules for naming silanes. For instance, depending on the number of silicon atoms in each molecule, the word silane is preceded by a numerical prefix, such as di, tri, tetra, and so forth. Thus, Si2H6 is disilane, and Si3H8 is trisilane. SiH4 is usually just called silane, without a prefix, but occasionally it is referred to as monosilane (to avoid confusion with larger silanes).
In an alternative system of nomenclature, silanes may be named in a manner similar to other inorganic compounds. For instance, silane is called silicon tetrahydride. However, with longer silanes, this system becomes cumbersome.
A cyclosilane is a silane with a ring structure, just as a cycloalkane is an alkane with a ring structure.
Some silanes have branched structures. The radical •SiH3 is termed silyl, •Si2H5 is disilanyl, and so on. Trisilane with a silyl group attached to the middle silicon is named silyltrisilane. The nomenclature parallels that of alkyl radicals.
Silanes may also carry certain functional groups, just as alkanes do. For instance, if a hydroxyl group (OH) is attached to a silane, it is called a silanol. There is (at least in principle) a silicon analog for all carbon alkanes.
Properties
As noted above, silane (SiH4) is a gas at room temperature. In addition, it is pyrophoric—that is, it undergoes spontaneous combustion in air, without the need for external ignition. (However, one school of thought holds that silane itself is stable and that the natural formation of larger silanes during production causes its pyrophoricity.) Above 420°C, silane decomposes into silicon and hydrogen. It can therefore be used in the chemical vapor deposition of silicon.
The family of silanes tends to be less stable than their carbon analogs because the Si-Si bond has a strength slightly lower than the C-C bond. Oxygen decomposes silanes easily, because the silicon-oxygen bond is quite stable.
Production
Industrially, silane is produced from metallurgical grade silicon in a two-step process. In the first step, powdered silicon is reacted with hydrochloric acid at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation:
- Si + 3HCl → HSiCl3 + H2
The trichlorosilane is then boiled on a resinous bed containing a catalyst, producing silane and silicon tetrachloride according to the chemical equation:
- 4HSiCl3 → SiH4 + 3SiCl4
The most commonly used catalysts for this process are metal halides, particularly aluminum chloride.
Applications
Silanes are useful for several industrial and medical applications. For instance, they are used as coupling agents to adhere glass fibers to a polymer matrix, stabilizing the composite material. They can also be used to couple a bio-inert layer on a titanium implant. Other applications include water repellents, masonry protection, control of graffiti,[1] applying polycrystalline silicon layers on silicon wafers when manufacturing semiconductors, and sealants. In addition, silane and similar compounds containing Si-H bonds are used as reducing agents in organic and organometallic chemistry.[2]
See also
Notes
- ↑ Graffiti protection systems Retrieved October 23, 2007.
- ↑ Reduction of organic compounds using silanes Retrieved October 23, 2007.
References
ISBN links support NWE through referral fees
- Antonucci, J.M., et al. 2003. Chemistry of Silanes: Interfaces in Dental Polymers and Composites Polymers Division, NIST. Retrieved August 23, 2007.
- Jutzi, Peter, and Ulrich Schubert. 2003. Silicon Chemistry: From the Atom to Extended Systems. New York: Wiley. ISBN 3527306471
- Plueddemann, Edwin Paul. 1982. Silane Coupling Agents. Berlin: Springer. ISBN 0306409577
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 06:28:02 | 41 views
☰ Source: https://www.newworldencyclopedia.org/entry/Silane | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF