Simes
 From Handwiki
From Handwiki 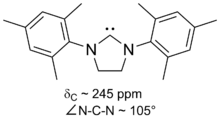
| |
| Names | |
|---|---|
| IUPAC name
1,3-Bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene
| |
| Other names
IMesH2, H2IMes, 1,3-Dimesityl-imidazol-4,5-dihydro-2-ylidene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C21H26N2 | |
| Molar mass | 306.453 g·mol−1 |
| Melting point | 79 to 85 °C (174 to 185 °F; 352 to 358 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
SIMes (or H2Imes) is an N-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry. It is structurally related to the more common ligand IMes but with a saturated backbone (the S of SIMes indicates a saturated backbone). It is slightly more flexible and is a component in Grubbs II.[1] It is prepared by alkylation of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation.[2]
References
- ↑ Nolan, Steven P. (2006). N-Heterocyclic Carbenes in Synthesis. Wiley-VCH. ISBN 978-3-527-60940-6.
- ↑ "Synthesis of 1,3–bis(2,4,6–trimethylphenyl)–imidazolinium salts : SIMes.HCl, SIMes.HBr, SIMes.HBF4 and SIMes.HPF6. : Protocol Exchange" (in en). http://www.nature.com/protocolexchange/protocols/2488#/procedure.
 |
Categories: [Carbenes]
↧ Download as ZWI file | Last modified: 03/09/2024 00:46:55 | 30 views
☰ Source: https://handwiki.org/wiki/Chemistry:SIMes | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF