Neodymium Bismuthide
 From Handwiki
From Handwiki 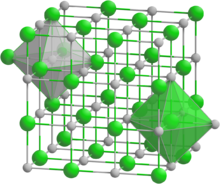
| |
| Names | |
|---|---|
| Other names
Neodymium(III) bismuthide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
BiNd |
| Molar mass | 352.22 g/mol |
| Density | 8.8 g/cm3 |
| Melting point | 1775°C;[1] 1900°C[2] |
| Critical point (T, P) | -111 kJ/mol[3] |
| Structure | |
Crystal structure
|
cubic |
Space group
|
Fm3m |
Lattice constant
|
a = 6.4222 Å
|
Formula units (Z)
|
4 |
| Related compounds | |
Other anions
|
Neodymium(III) nitride Neodymium(III) arsenide Neodymium(III) phosphide Neodymium(III) antimonide Neodymium(III) oxide |
Other cations
|
PrBi |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Neodymium bismuthide or Bismuth-Neodymium[4] is a binary inorganic compound of neodymium and bismuth with the formula NdBi. It forms crystals.
Preparation
Neodymium bismuthide can be prepared by reacting a stoichiometric amount of neodymium and bismuth at 1900°C:[citation needed]
- Nd + Bi → NdBi
Physical properties
Neodymium bismuthide forms cubic crystals of the space group Fm3m, with cell parameters a = 0.64222 nm, Z = 4 with a structure like sodium chloride.[5] The compound melts at 1900°C.[2] At a temperature of 24 K, an antiferromagnetic transition occurs in the compound.[6]
References
- ↑ Abulkhaev V.D. (2001). "Phase digram of the neodymium-bismuth system". Zhurnal Neorganicheskoj Khimii 46 (4): 659-662. ISSN 0044-457X. https://inis.iaea.org/search/search.aspx?orig_q=RN:33016098.
- ↑ 2.0 2.1 Alloy Phase Diagrams. 3. 1992. ISBN 0-87170-381-5.
- ↑ A. Borsese; R. Capelli; S. Delfino; R. Ferro (1974). "The heat of formation of neodymium-bismuth alloys". Thermochimica Acta 8 (4): 393-397. doi:10.1016/0040-6031(74)85107-5.
- ↑ Okamoto, H. (2002-03-01). "Bi-Nd (Bismuth-Neodymium)" (in en). Journal of Phase Equilibria 23 (2): 191. doi:10.1361/1054971023604224. ISSN 1054-9714. https://doi.org/10.1361/1054971023604224.
- ↑ Ed. N. P. Lyakisheva (1996). State Diagrams of Binary Metal Systems. 1. Engineering. p. 992. ISBN 5-217-02688-X.
- ↑ P. Schobinger-Papamantellos, P. Fischer, O. Vogt, E. Kaldis (1973). "Magnetic ordering of neodymium monopnictides determined by neutron diffraction". J. Phys. C: Solid State Phys. 6 (4): 725-737. doi:10.1088/0022-3719/6/4/020. Bibcode: 1973JPhC....6..725S.
 |
Categories: [Neodymium(III) compounds] [Bismuth compounds] [Rock salt crystal structure]
↧ Download as ZWI file | Last modified: 01/27/2025 01:04:19 | 9 views
☰ Source: https://handwiki.org/wiki/Chemistry:Neodymium_bismuthide | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF