Periodic Table
 From Nwe
From Nwe The periodic table of the chemical elements is a tabular display of the chemical elements. It is perhaps the icon of Chemistry and expresses much about the physical and chemical properties of the known elements. The emergence of the periodic table occurred concurrently with the development of the scientific understanding of the composition of matter. In its current form, it owes much to quantum mechanics. The electronic structures derived from quantum mechanics are used to explain theoretically the experimentally observed periodic variations in properties of the elements. The periodic table is one of the essential components of our understanding of the universe and underlies all of chemistry.
History
- Main article: History of the periodic table
The original table was created without a knowledge of the inner structure of atoms, but rather by correlating physical and chemical properties of the elements with atomic mass. If the elements are ordered by atomic mass then a certain periodicity, or regular repetition, of physical and chemical properties can be observed. The first to recognize these regularities was the German chemist Johann Wolfgang Döbereiner who, in 1829, noticed a number of triads of similar elements:
| Element | Molar mass (g/mol) |
Density (g/cm³) |
Quotient (cm³/mol) |
|---|---|---|---|
| chlorine | 35.4527 | 0.003214 | 11030 |
| bromine | 79.904 | 3.122 | 25.6 |
| iodine | 126.90447 | 4.93 | 25.7 |
| calcium | 40.078 | 1.54 | 26.0 |
| strontium | 87.62 | 2.64 | 33.2 |
| barium | 137.327 | 3.594 | 38.2 |
This was followed by the English chemist John Newlands, who noticed in 1865 that the elements of similar type recurred at intervals of eight, which he likened to the octaves of music, though his law of octaves was ridiculed by his contemporaries. Finally, in 1869, the German Julius Lothar Meyer and the Russian chemistry professor Dmitri Ivanovich Mendeleev almost simultaneously developed the first periodic table, arranging the elements by mass. However, Mendeleev plotted a few elements out of strict mass sequence in order to make a better match to the properties of their neighbors in the table. He also corrected mistakes in the values of several atomic masses, and predicted the existence and properties of a few new elements in the empty cells of his table. Mendeleev was later vindicated by the discovery of the electronic structure of the elements in the late nineteenth century and early twentieth century. The modern table is based on this understanding of the electronic structures.
In 1913, Henry Moseley rearranged the table according to atomic number to improve the observed periodicity in the chemical properties across the table. Today's table uses this ordering by atomic number (number of protons). Mendeleev's and Moseley's development of the periodic table was one of the greatest achievements in modern chemistry. Chemists were able to qualitatively explain the behavior of the elements, and to predict the existence of yet undiscovered ones.
In the 1940s Glenn T. Seaborg identified the transuranic lanthanides and the actinides, which may be placed within the table, or below (see the different possible arrangements below).
Methods for displaying the periodic table
Standard periodic table
| Group → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period ↓ | ||||||||||||||||||||
| 1 | 1 H |
2 He |
||||||||||||||||||
| 2 | 3 Li |
4 Be |
5 B |
6 C |
7 N |
8 O |
9 F |
10 Ne |
||||||||||||
| 3 | 11 Na |
12 Mg |
13 Al |
14 Si |
15 P |
16 S |
17 Cl |
18 Ar |
||||||||||||
| 4 | 19 K |
20 Ca |
21 Sc |
22 Ti |
23 V |
24 Cr |
25 Mn |
26 Fe |
27 Co |
28 Ni |
29 Cu |
30 Zn |
31 Ga |
32 Ge |
33 As |
34 Se |
35 Br |
36 Kr |
||
| 5 | 37 Rb |
38 Sr |
39 Y |
40 Zr |
41 Nb |
42 Mo |
43 Tc |
44 Ru |
45 Rh |
46 Pd |
47 Ag |
48 Cd |
49 In |
50 Sn |
51 Sb |
52 Te |
53 I |
54 Xe |
||
| 6 | 55 Cs |
56 Ba |
* |
72 Hf |
73 Ta |
74 W |
75 Re |
76 Os |
77 Ir |
78 Pt |
79 Au |
80 Hg |
81 Tl |
82 Pb |
83 Bi |
84 Po |
85 At |
86 Rn |
||
| 7 | 87 Fr |
88 Ra |
** |
104 Rf |
105 Db |
106 Sg |
107 Bh |
108 Hs |
109 Mt |
110 Ds |
111 Rg |
112 Uub |
113 Uut |
114 Uuq |
115 Uup |
116 Uuh |
117 Uus |
118 Uuo |
||
| * Lanthanides | 57 La |
58 Ce |
59 Pr |
60 Nd |
61 Pm |
62 Sm |
63 Eu |
64 Gd |
65 Tb |
66 Dy |
67 Ho |
68 Er |
69 Tm |
70 Yb |
71 Lu |
|||||
| ** Actinides | 89 Ac |
90 Th |
91 Pa |
92 U |
93 Np |
94 Pu |
95 Am |
96 Cm |
97 Bk |
98 Cf |
99 Es |
100 Fm |
101 Md |
102 No |
103 Lr |
|||||
| Alkali metals | Alkaline earth metals | Lanthanides | Actinides | Transition metals |
| Poor metals | Metalloids | Nonmetals | Halogens | Noble gases |
State at standard temperature and pressure
- Elements numbered in red are gases.
- Elements numbered in green are liquids.
- Elements numbered in black are solids.
Natural occurrence
-
Elements without borders have not been discovered/synthesized yet.
-
Elements with dotted borders do not occur naturally (synthetic elements).
-
Elements with dashed borders naturally arise from decay of other chemical elements.
-
Elements with solid borders are older than the Earth (primordial elements).
- Note: Although californium (Cf, 98) is not Earth-primordial, it (and its decay products) does occur naturally: its electromagnetic emissions are regularly observed in supernova spectra.
Other depictions
- The standard table (shown above) provides the basics.
- A vertical table for improved readability in web browsers.
- The large table provides the basics plus full element names and atomic masses.
- A table with an inline F-block inserts the lanthanides and actinides back into the table.
- Electron configurations
- Metals and non-metals
- Periodic table filled by blocks
- List of elements by name with atomic number and atomic mass
- List of elements by electronegativity
- Mendeleev's periodic table
Structure of the Table
Each element appears in a box which contains the symbol of the element and its atomic number. Many tables also include the atomic mass, and some have additional information as well. The fundamental ordering of the elements is as a list according to their atomic number (number of protons). As of 2005, the table contains 116 chemical elements whose discoveries have been confirmed. Of those 94 are found naturally on Earth, and the rest are synthetic elements that have been produced artificially in laboratories. Following this basic order the elements are arranged in a table that contains specific columns and rows, known as groups and periods respectively (see the above table).
Groups
The columns of the table are known as groups or families. All the elements in a group have similar properties. Placing elements in groups is one of the most important ways of classifying them. There is some variation in properties within a group, but the changes are relatively small as one goes down (or up) the group. Each group of elements forms what is called a chemical series.
There are three ways of numbering the groups of the periodic table. The standard International Union of Pure and Applied Chemistry (IUPAC) system is to simply number them 1 though 18 as in the table above. There are also two older systems using Roman numerals. The Roman numeral names are the original traditional names of the groups; the standard IUPAC system replaces the old names in an attempt to reduce the confusion generated by the two older, but mutually confusing, schemes. Some of the groups have special names (see below). Groups 1, 2, 13, 14, 15, 16, 17, and 18 are also collectively known as the main group, or representative, elements, and groups 3 through 12 are the transition metals.
There is considerable confusion surrounding the two old systems in use (old IUPAC and CAS) that combined the use of Roman numerals with letters. In the old IUPAC system the letters A and B were designated to the left (A) and right (B) part of the table, while in the CAS system the letters A and B were designated to main group elements (A) and transition metals (B). The former system was frequently used in Europe while the latter was most common in America. The new IUPAC scheme was developed to replace both systems as they confusingly used the same names to mean different things.
The periodic table groups are as follows (in the brackets are shown the old systems: European and American):
- Group 1 (IA,IA): the alkali metals
- Group 2 (IIA,IIA): the alkaline earth metals
- Group 3 (IIIA,IIIB)
- Group 4 (IVA,IVB)
- Group 5 (VA,VB)
- Group 6 (VIA,VIB)
- Group 7 (VIIA,VIIB)
- Group 8 (VIII)
- Group 9 (VIII)
- Group 10 (VIII)
- Group 11 (IB,IB): the coinage metals (not a IUPAC-recommended name)
- Group 12 (IIB,IIB)
- Group 13 (IIIB,IIIA): the boron group
- Group 14 (IVB,IVA): the carbon group
- Group 15 (VB,VA): the pnictogens (not a IUPAC-recommended name) or nitrogen group
- Group 16 (VIB,VIA): the chalcogens
- Group 17 (VIIB,VIIA): the halogens
- Group 18 (Group 0): the noble gases
Periods
The rows of the table are known as periods. It is in the succesive periods that we observe the periodicity of properties of the elements. Each period has the full range of properties. For example more metallic elements occur to the left of a period, and the less metallic elements to the right; or oxides of the elements to the left are basic and acidic for elements to the right. The periods are simply numbered 1 though 7 from the top down
Electronic structure
The shape of the periodic table and the placement of an element in a particular group or period is derived from the electronic structure of the atoms of the element. In fact the chemical and physical properties of an element derive from it's electronic structure. Thus it is the electronic structures of the elements that are the source of the observed periodicity of properties and the groups and periods of the periodic table.
The electronic structures of the elements derive from quantum mechanics. The quantum mechanical description of an atom suggests that the electrons have a complex, but precise organization surrounding the atomic nucleus. The electrons are organized primarily into shells of increasing size and energy, which are numbered sequentially beginning with 1 as the lowest energy. The shells contain subshells which can be represented by letters. The most common subshells are s, p, and d. The subshells are in turn comprised of orbitals, where each orbital can contain two electrons.
Of particular importance are the electrons in the highest energy (outermost) shell. These are the electrons that determine the position of the element in the table and are primarily responsible for the properties of the element. In the main group elements these outermost electrons are known as the valence electrons. The elements in a given group all have the same number of valence electrons, but they reside in successively higher shells as you go down the group. This is what gives the elements in a group similar properties. For example all the main group elements with four valence electrons are in Group 14 starting with carbon. They all have their valence electrons in s and p subshells. Those four s and p electrons will behave similarly regardless of shell they are in.
In addition to dividing the table into groups and periods the table can be divided into blocks (see Periodic table filled by blocks) where the last subshell in which the atom's outermost electrons reside determines the "block" to which it belongs. Carbon, for example, is in the p-block because its last electrons are in the p subshell.
The total number of electron shells an atom has determines the period to which it belongs. Since each shell is divided into different subshells, as we step through the elements by atomic number, the subshells will fill with electrons roughly in the order shown in the table below (in the table the numbers refer to the shell and the letters to the subshell):
| Subshell: | S | G | F | D | P |
| Period | |||||
| 1 | 1s | ||||
| 2 | 2s | 2p | |||
| 3 | 3s | 3p | |||
| 4 | 4s | 3d | 4p | ||
| 5 | 5s | 4d | 5p | ||
| 6 | 6s | 4f | 5d | 6p | |
| 7 | 7s | 5f | 6d | 7p | |
| 8 | 8s | 5g | 6f | 7d | 8p |
Hence the structure of the table. Since the outermost electrons determine chemical properties, those with the same number of valence electrons are grouped together.
See also
- Atom
- Atomic mass
- Atomic number
- Chemical element
- Electron configuration
- Isotope
- Metal
- Nonmetal
- Transition element
References
ISBN links support NWE through referral fees
- Bouma, J. 1989. An Application-Oriented Periodic Table of the Elements. J. Chem. Ed. 66:741.
- Cotton, F. Albert, G. Wilkinson, C.A. Murillo, and M. Bochmann. 1999. Advanced Inorganic Chemistry, 6th ed. New York: Wiley. ISBN 0471199575
- Greenwood, N. N., and A. Earnshaw. 1997. Chemistry of the Elements, 2nd ed. Oxford: Butterworth-Heinemann. ISBN 0750633654
- Mazurs, Edward G. 1974. Graphic Representations of the Periodic System During One Hundred Years. University, AL: University of Alabama Press. ISBN 0817332006
- Scerri, Eric R. 2007. The Periodic Table: Its Story and Its Significance. Oxford: Oxford University Press. ISBN 978-0195305739
External links
All links retrieved November 23, 2022.
- Periodic table. WebElements.
- Periodic Table. Large full-color scalable.
|
||||||||
| Standard table | Vertical table | Table with names | Names and atomic masses (large) | Names and atomic masses (small) | Names and atomic masses (text only) | Inline F-block | Elements to 218 | Electron configurations | Metals and non metals | Table by blocks | List of elements by name |
| Groups: 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 - 9 - 10 - 11 - 12 - 13 - 14 - 15 - 16 - 17 - 18 |
| Periods: 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 |
| Series: Alkalis - Alkaline earths - Lanthanides - Actinides - Transition metals - Poor metals - Metalloids - Nonmetals - Halogens - Noble gases |
| Blocks: s-block - p-block - d-block - f-block - g-block |
| General subfields within the Natural sciences |
|---|
| Astronomy | Biology | Chemistry | Earth science | Ecology | Physics |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/03/2023 20:16:55 | 279 views
☰ Source: https://www.newworldencyclopedia.org/entry/Periodic_table | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: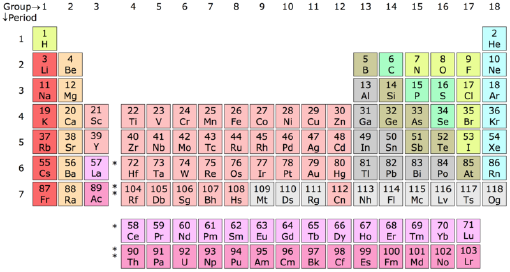
 KSF
KSF