Butyl Nitrate
 From Handwiki
From Handwiki 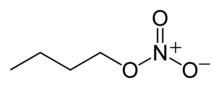
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
Butyl nitrate | |
| Other names
Nitric acid butyl ester; 1-Nitrooxy-butane
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C4H9NO3 |
| Molar mass | 119.120 g·mol−1 |
| Appearance | Colorless oil |
| Density | 1.047 g/cm3 |
| Melting point | 0 °C (32 °F; 273 K) |
| Boiling point | 133 °C (271 °F; 406 K) |
Solubility in water
|
1120 mg/L |
| Vapor pressure | 9.6 mmHg |
| Hazards | |
| GHS pictograms | 
|
| Flash point | 49.9 °C (121.8 °F; 323.0 K) |
| Related compounds | |
Related hydrocarbons
|
Cyclopentanone |
Related compounds
|
nitric acid, butyl ester |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Butyl nitrate is a colorless oil. It is often confused with butyl nitrite, which is sometimes used as a recreational inhalant.[1]
Safety
Butyl nitrate is an explosive. It reacts explosively with Lewis acids such as boron trifluoride and aluminium chloride. When heated to decomposition, it emits toxic fumes of nitrous oxide.[2]
References
- ↑ "Butyl Nitrite Drug Profile". DAODAS. 11 October 2012. http://www.daodas.state.sc.us/profile_butyl-nitrite.asp.
- ↑ Fact Sheet 30
This article includes a list of references, but its sources remain unclear because it has insufficient inline citations. (February 2016) (Learn how and when to remove this template message) |
- "Nitric Acid, Butylester." Butyl Nitrate (928-45-0),Butyl Nitrate (928-45-0) Manufacturers & Suppliers,Synthesis,MSDS. N.p., n.d. Web. 11 Oct. 2012.
- "Butyl nitrite transformation in vitro, chemical nitrosation reactions, and mutagenesis". Journal of Analytical Toxicology 8 (4): 164–9. 1984. doi:10.1093/jat/8.4.164. PMID 6471815.
Categories: [Alkyl nitrates]
↧ Download as ZWI file | Last modified: 07/30/2022 19:08:23 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Butyl_nitrate | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF