Ether
 From Nwe
From Nwe - This article is about a general class of chemical compounds. For other uses, see Aether.
Ether is the general name for a class of organic chemical compounds characterized by molecules that contain an ether functional group—an oxygen atom directly bound to two hydrocarbon (alkyl or aryl) groups. A typical example is diethyl ether, commonly known as "ether" (ethoxyethane, CH3-CH2-O-CH2-CH3). Different ethers have diverse uses, but most are useful as solvents. Dimethyl ether is a multi-purpose fuel, refrigerant, aerosol spray propellant, and a medium for chemical reactions. Ethylene glycol is used in the production of various chemicals and to sterilize medical supplies and spices. Diethyl ether has been used as an anesthetic; dimethoxyethane is used in organometallic chemistry; dioxane is a foaming agent; tetrahydrofuran is used to degrease metal parts; anisole is used in perfumes and as an insect pheromone; and crown ethers are used to hold metal cations in solution. Polyethylene glycol, a polymeric ether, is used in laxatives, skin creams, toothpastes, and various medications.
Nomenclature
Trivial names and IUPAC names
The traditional approach has been to name the two alkyl groups attached to the oxygen atom (of the ether) and to append "ether" at the end. Examples are "ethyl methyl ether" and "diethyl ether." These are called "trivial names."
In the IUPAC nomenclature system, ethers are named using the general formula, "alkoxyalkane." For example, CH3-CH2-O-CH3 is methoxyethane. If the ether is part of a more complex molecule, it is described as an alkoxy substituent, so -OCH3 would be considered a "methoxy-" group.
Primary, secondary, and tertiary ethers
The ether may be classified as a "primary ether," "secondary ether," or "tertiary ether," depending on the substituents on the carbon atoms next to the ether oxygen. For example, diethyl ether, CH3-CH2-O-CH2-CH3, is called a primary ether because each carbon atom attached to the ether oxygen atom is directly linked to only one other carbon atom. An example of a secondary ether is diisopropyl ether, (CH3)2CH-O-CH(CH3)2, in which each carbon atom attached to the ether oxygen atom is directly linked to two other carbon atoms. An example of a tertiary ether is di-tert-butyl ether, (CH3)3C-O-C(CH3)3, in which each carbon atom attached to the ether oxygen atom is directly linked to three other carbon atoms.
Top to bottom: Dimethyl ether; a primary ether (diethyl ether); a secondary ether (diisopropyl ether); and a tertiary ether (di-tert-butyl ether).
Polyethers
Polyethers are compounds with more than one ether group. The term is generally used when referring to polymers such as polyethylene glycol and polypropylene glycol. It is also used for low molecular weight compounds such as the crown ethers.
Compounds with similar structures
Ethers are not to be confused with other classes of compounds with the same general structure R-O-R'. Some examples are given below.
- Aromatic compounds like furan, where an oxygen atom is part of the aromatic system.
- Compounds where a carbon atom next to the oxygen is connected to oxygen, nitrogen, or sulfur:
- Esters R'-C(=O)-O-R
- Acetals R'-CH(-O-R)-O-R
- Aminals R'-CH(-NH-R)-O-R
- Anhydrides R'-C(=O)-O-C(=O)-R
- Thionoesters R'-C(=S)-O-R
Physical properties
Ether molecules cannot form hydrogen bonds with each other, resulting in a relatively low boiling point comparable to that of the analogous alcohols. However, the differences in the boiling points of the ethers and their isometric alcohols become smaller as the carbon chains become longer, as the hydrophobic nature of the carbon chain becomes more predominant over the presence of hydrogen bonding.
Ethers are slightly polar as the C-O-C bond angle in the functional group is about 110 degrees, and the C-O dipole does not cancel out. Ethers are more polar than alkenes but not as polar as alcohols, esters, or amides of comparable structure. However, the presence of two lone pairs of electrons on the oxygen atoms makes hydrogen bonding with water molecules possible, causing the solubility of alcohols (for instance, butan-1-ol) and ethers (ethoxyethane) to be quite dissimilar.
Cyclic ethers such as tetrahydrofuran and 1,4-dioxane are totally miscible in water because of the more exposed oxygen atom for hydrogen bonding as compared to aliphatic ethers.
Ethers can act as Lewis bases. For instance, diethyl ether forms a complex with boron compounds, such as boron trifluoride diethyl etherate (BF3.OEt2). Ethers also coordinate to magnesium in Grignard reagents (RMgBr).
Chemical reactions
Ethers are generally low in chemical reactivity. Some of their reactions are as follows.
- Ethers are hydrolyzed only under drastic conditions like heating with boron tribromide or boiling in hydrobromic acid. Lower mineral acids containing a halogen, such as hydrochloric acid will cleave ethers, but very slowly. Hydrobromic acid and hydroiodic acid are the only two that do so at an appreciable rate. Certain aryl ethers can be cleaved by aluminum chloride.
- Nucleophilic displacement.
- Epoxides, or cyclic ethers in three-membered rings, are highly susceptible to nucleophilic attack and are reactive in this fashion.
- Peroxide formation.
- Primary and secondary ethers with a CH group next to the ether oxygen easily form highly explosive organic peroxides (e.g. diethyl ether peroxide) in the presence of oxygen, light, and metal and aldehyde impurities. For this reason, ethers like diethyl ether and THF are usually avoided as solvents in industrial processes.
Syntheses
Ethers can be prepared in the laboratory in several different ways.
- Intermolecular Dehydration of alcohols:
- R-OH + R-OH → R-O-R + H2O
- This direct reaction requires drastic conditions (heating to 140 degrees Celsius and an acid catalyst, usually concentrated sulphuric acid). Effective for making symmetrical ethers, but not as useful for synthesizing asymmetrical ethers, because the reaction will yield a mixture of ethers, usually making it not applicable:
- 3R-OH + 3R'-OH → R-O-R + R'-O-R + R'-O-R' + 3H2O
- Conditions must also be controlled to avoid overheating to 170 degrees, which will cause intramolecular dehydration, a reaction that yields alkenes. In addition, the alcohol must be in excess.
- R-CH2-CH2(OH) → R-CH=CH2 + H2O
- Such conditions can destroy the delicate structures of some functional groups. There exist several milder methods to produce ethers.
- Nucleophilic displacement of alkyl halides by alkoxides
- R-O- + R-X → R-O-R + X-
- This reaction is called the Williamson ether synthesis. It involves treatment of a parent alcohol with a strong base to form the alkoxide anion, followed by addition of an appropriate aliphatic compound bearing a suitable leaving group (R-X). Suitable leaving groups (X) include iodide, bromide, or sulfonates. This method does not work if R is aromatic, like in bromobenzene (Br-C6H5), however, if the leaving group is separated by at least one carbon from the benzene, the reaction should proceed (as in Br-CH2-C6H5). Likewise, this method only gives the best yields for primary carbons, as secondary and tertiary carbons will undergo E2 elimination on exposure to the basic alkoxide anion used in the reaction due to steric hindrance from the large alkyl groups. Aryl ethers can be prepared in the Ullmann condensation.
- Nucleophilic Displacement of Alkyl halides by phenoxides
- As mentioned above, when one of the R groups in the target ether is aromatic, the R-X cannot be used to react with the alcohol. However, phenols can be used to replace the alcohol, while maintaining the alkyl halide. Since phenols are acidic, they readily react with a strong base, like sodium hydroxide, to form phenoxide ions. The phenoxide ion will then substitute the -X group in the alkyl halide, forming an ether with an aryl group attached to it.
- HO-C6H5 + OH- → O--C6H5
- O--C6H5 + R-X → R-O-C6H5
- Electrophilic addition of alcohols to alkenes.
- R2C=CR2 + R-OH → R2CH-C(-O-R)-R2
- Acid catalysis is required for this reaction. Tetrahydropyranyl ethers are used as protective groups for alcohols.
Cyclic ethers, also known as epoxides, can be prepared by these methods:
- The oxidation of alkenes with a peroxy acid such as m-CPBA.
- The base intramolecular nucleophilic substitution of a halohydrin.
Important ethers and their uses
| Ethylene oxide | It is the smallest cyclic ether and is mainly used as an intermediate in the production of ethylene glycol and other chemicals. It is also used to sterilize medical supplies and spices. | |
 |
Dimethyl ether | It is useful as a solvent (in liquefied form), multi-purpose fuel, refrigerant, aerosol spray propellant, medium for chemical reactions, and a blowing agent for foam.[1] |
 |
Diethyl ether | It is a common solvent for organic compounds, and it has been used as a general anesthetic. |
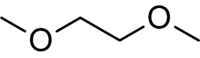 |
Dimethoxyethane | It is a good solvent and a higher boiling alternative to diethyl ether and tetrahydrofuran. It is often used in organometallic chemistry and is the low-viscosity component of the solvent for electrolytes in lithium batteries. |
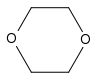 |
1,4-Dioxane | This cyclic ether is used mainly as a solvent in industry, but it is also a foaming agent and is present in fumigants and automotive coolants. |
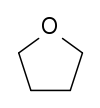 |
Tetrahydrofuran (THF) | This cyclic ether is one of the most polar simple ethers used as a solvent. It is also used to degrease metal parts. |
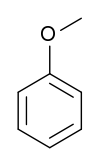 |
Anisole (methoxybenzene) | This aryl ether is a major constituent of the essential oil of anise seed. It is used in perfumes and as an insect pheromone. |
| Crown ethers | These cyclic polyethers can form chemical complexes with metal cations (such as lithium, sodium, and potassium cations), thus holding the cations in solution. The size of the interior of the crown ether determines the size of the cation it can solvate. | |
| Polyethylene glycol (PEG) | This nontoxic, water-soluble polymer is the basis for a number of laxatives and skin creams, and is a dispersant in various toothpastes. It prolongs the medicinal effect of protein medications, when the proteins are attached to it. In woodworking, it can be used to replace some of the water content in wood, to prevent the wood from warping or shrinking when dried. |
Similar terms, dissimilar meanings
Mythology:
- Aether (mythology): In Greek mythology, aether (or ether) was the personification of the "upper sky," space, and heaven.
Early science and philosophy:
- Aether (classical element): In ancient and medieval science, aether (or ether) was thought of as a substance that filled the region of the universe above the terrestrial sphere. Aristotle considered it to be the fifth element, distinct from Air, Fire, Earth, and Water.
- Luminiferous aether (or luminiferous ether): This term, meaning "light-bearing ether," was postulated to exist in outer space as the medium for the propagation of light. From the early twentieth century, scientific theories have been formulated without the concept of this type of ether.
- Etheric plane: It was thought of as a finer grade of matter—in addition to solids, liquids, and gases—that permeates the subatomic structure of the Earth and its atmosphere.
- Etheric body: A sort of life-force body or aura that constitutes the "blueprint" of the physical body and sustains the physical body.
Modern chemistry:
- Petroleum ether: This term is used for a low-boiling mixture of hydrocarbons, although chemically it does not contain any ether.
- Thioether: This is the general term for analogs of ethers in which the oxygen atom (that characterizes an ether) is replaced by a sulfur atom.
Notes
- ↑ Akzo Nobel, Demeon® D or Dimethyl Ether. Retrieved June 10, 2007.
References
ISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052
- Morrison, Robert T. and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0-13-643669-2
- Solomons, T.W. Graham and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998
External links
All links retrieved August 15, 2017.
- Ether. ILPI.com.
| Functional groups |
|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioether • Thiol • Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
- Ether history
- Ethylene_oxide history
- Dimethoxyethane history
- 1,4-Dioxane history
- Tetrahydrofuran history
- Anisole history
- Crown_ether history
- Polyethylene_glycol history
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/03/2023 22:20:52 | 97 views
☰ Source: https://www.newworldencyclopedia.org/entry/Ether | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:

 KSF
KSF