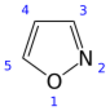
Isoxazole
 From Handwiki
From Handwiki
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Oxazole[1] | |||
| Other names
isoxazole
| |||
| Identifiers | |||
CAS Number
|
| ||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChEMBL |
| ||
| ChemSpider |
| ||
PubChem CID
|
| ||
| UNII |
| ||
InChI
| |||
SMILES
| |||
| Properties | |||
Chemical formula
|
C3H3NO | ||
| Molar mass | 69.06202 g/mol | ||
| Density | 1.075 g/ml | ||
| Boiling point | 95 °C (203 °F; 368 K) | ||
| Acidity (pKa) | -3.0 (of conjugate acid)[2] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Isoxazole is an electron-rich azole with an oxygen atom next to the nitrogen. It is also the class of compounds containing this ring. Isoxazolyl is the univalent radical derived from isoxazole.
Occurrence
Isoxazole rings are found in some natural products, such as ibotenic acid and muscimol.
Synthesis
Isoxazole can be synthesised via a variety of methods.[3][4] Examples include via a 1,3-dipolar cycloaddition of nitrile oxides with alkynes; or the reaction of hydroxylamine with 1,3-diketones or derivatives of propiolic acid.[5]
Pharmaceuticals and herbicides
Isoxazoles also form the basis for a number of drugs,[6] including the COX-2 inhibitor valdecoxib (Bextra) and a neurotransmitter agonist AMPA. A derivative, furoxan, is a nitric oxide donor. An isoxazolyl group is found in many beta-lactamase-resistant antibiotics, such as cloxacillin, dicloxacillin and flucloxacillin. Leflunomide is an isoxazole-derivative drug. Examples of AAS containing the isoxazole ring include danazol and androisoxazole. A number of pesticides are isoxazoles.[7] thumb|left|Isoxaben is an example of an isoxazole used as a herbicide.
See also
- Oxazole, an analog with the nitrogen atom in position 3
- Pyrrole, an analog without the oxygen atom
- Furan, an analog without the nitrogen atom
- Simple aromatic rings
References
- ↑ International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. pp. 140. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ↑ Zoltewicz, J. A. & Deady, L. W. Quaternization of heteroaromatic compounds. Quantitative aspects. Adv. Heterocycl. Chem. 22, 71-121 (1978)
- ↑ "An overview of metal-free synthetic routes to isoxazoles: the privileged scaffold". RSC Adv (11): 32680-32705. 2021. doi:10.1039/D1RA04624A.
- ↑ Morita, Taiki; Yugandar, Somaraju; Fuse, Shinichiro; Nakamura, Hiroyuki (March 2018). "Recent progresses in the synthesis of functionalized isoxazoles". Tetrahedron Letters 59 (13): 1159–1171. doi:10.1016/j.tetlet.2018.02.020.
- ↑ "Synthesis of 3,4,5-trisubstituted isoxazoles in water via a [3 + 2]-cycloaddition of nitrile oxides and 1,3-diketones, β-ketoesters, or β-ketoamides". Beilstein J. Org. Chem. (18): 446–458. 2022. doi:10.3762/bjoc.18.47. PMID 35529890.
- ↑ Zhu, Jie; Mo, Jun; Lin, Hong-zhi; Chen, Yao; Sun, Hao-peng (2018). "The recent progress of isoxazole in medicinal chemistry". Bioorganic & Medicinal Chemistry 26 (12): 3065–3075. doi:10.1016/j.bmc.2018.05.013.
- ↑ Clemens Lamberth (2018). "Oxazole and Isoxazole Chemistry in Crop Protection". Journal of Heterocyclic Chemistry 55 (9): 2035–2045. doi:10.1002/jhet.3252.
External links
- Synthesis of isoxazoles (overview of recent methods)
 |
Categories: [Isoxazoles] [Simple aromatic rings]
↧ Download as ZWI file | Last modified: 01/05/2023 04:51:44 | 2 views
☰ Source: https://handwiki.org/wiki/Chemistry:Isoxazole | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed: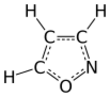

 KSF
KSF