Lactones
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Lactones, the cyclic esters of hydroxy acids, resulting from
the internal elimination of water between the hydroxyl and
carboxyl groups, this reaction taking place when the hydroxy
acid is liberated from its salts by a mineral acid. The α and β-hydroxy
acids do not form lactones, the tendency for lactone
formation appearing first with the γ-hydroxy acids, thus γ-hydroxybutyric
acid, CH2OH·CH2·CH2·CO2H, yields γ-butyrolactone,
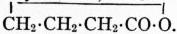 These compounds may also be
prepared by the distillation of the γ-halogen fatty acids, or by
the action of alkaline carbonates on these acids, or from βγ- or
γδ-unsaturated acids by digestion with hydrobromic acid or
dilute sulphuric acid. The lactones are mostly liquids which
are readily soluble in alcohol, ether and water. On boiling
with water, they are partially reconverted into the hydroxy acids.
They are easily saponified by the caustic alkalis.
These compounds may also be
prepared by the distillation of the γ-halogen fatty acids, or by
the action of alkaline carbonates on these acids, or from βγ- or
γδ-unsaturated acids by digestion with hydrobromic acid or
dilute sulphuric acid. The lactones are mostly liquids which
are readily soluble in alcohol, ether and water. On boiling
with water, they are partially reconverted into the hydroxy acids.
They are easily saponified by the caustic alkalis.
On the behaviour of lactones with ammonia, see H. Meyer, Monatshefte, 1899, 20, p. 717; and with phenylhydrazine and hydrazine hydrate, see R. Meyer, Ber., 1893, 26, p. 1273; L. Gattermann, Ber., 1899, 32, p. 1133, E. Fischer, Ber., 1889, 22, p. 1889.
γ-Butyrolactone is a liquid which boils at 206° C. It is miscible
with water in all proportions and is volatile in steam,
γ-valerolactone,
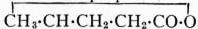 ,
is a liquid which boils at 207-208°
C. δ-lactones are also known, and may be prepared by distilling
the δ-chlor acids.
,
is a liquid which boils at 207-208°
C. δ-lactones are also known, and may be prepared by distilling
the δ-chlor acids.
↧ Download as ZWI file | Last modified: 11/17/2022 15:23:02 | 27 views
☰ Source: https://oldpedia.org/article/britannica11/Lactones | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF