Zirconium Tetrafluoride
 From Handwiki
From Handwiki -fluorid.png)
| |
| Names | |
|---|---|
| IUPAC names
Zirconium(IV) fluoride
Zirconium tetrafluoride | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
ZrF4 |
| Molar mass | 167.21 g/mol |
| Appearance | white crystalline powder |
| Density | 4.43 g/cm3 (20 °C) |
| Melting point | 910 °C (1,670 °F; 1,180 K) |
Solubility in water
|
1.32 g/100mL (20 °C) 1.388 g/100mL (25 °C) |
| Structure | |
Crystal structure
|
Monoclinic, mS60 |
Space group
|
C12/c1, No. 15 |
| Hazards | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
98 mg/kg (oral, mouse) 98 mg/kg (oral, rat)[1] |
| Related compounds | |
Other anions
|
Zirconium(IV) chloride Zirconium(IV) bromide Zirconium(IV) iodide |
Other cations
|
Titanium(IV) fluoride Hafnium(IV) fluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zirconium(IV) fluoride describes members of a family inorganic compounds with the formula (ZrF4(H2O)x. All are colorless, diamagnetic solids. Anhydrous Zirconium(IV) fluoride' is a component of ZBLAN fluoride glass.[2]
Structure
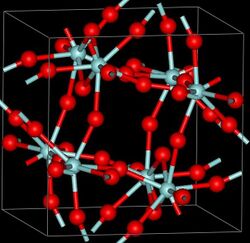
Three crystalline phases of ZrF4 have been reported, α (monoclinic), β (tetragonal, Pearson symbol tP40, space group P42/m, No 84) and γ (unknown structure). β and γ phases are unstable and irreversibly transform into the α phase at 400 °C.[3]
Zirconium(IV) fluoride forms several hydrates. The trihydrate has the structure (μ–F)
2[ZrF
3(H
20)
3]
2.[4]
Preparation and reactions
Zirconium fluoride can be produced by several methods. Zirconium dioxide reacts with hydrogen fluoride and hydrofluoric acid to afford the anhydrous and monohydrates:
- ZrO
2 + 4 HF → ZrF
4 + 2 H
2O
The reaction of Zr metal reacts at high temperatures with HF as well:
- Zr + 4 HF → ZrF
4 + 2 H
2
Zirconium dioxide reacts at 200 °C with solid ammonium bifluoride to give the heptafluorozirconate salt, which can be converted to the tetrafluoride at 500 °C:
- 2ZrO
2 + 7 (NH
4)HF
2 → 2 (NH
4)
3ZrF
7 + 4 H
2O + NH
3 - (NH
4)
3ZrF
7 → ZrF
4 + 3 HF + 3 NH
3
Addition of hydrofluoric acid to solutions of zirconium nitrate precipitates solid monohydrate. Hydrates of zirconium tetrafluoride can be dehydrated by heating under a stream of hydrogen fluoride.
Zirconium fluoride can be purified by distillation or sublimation.[2]
Zirconium fluoride forms double salts with other fluorides. The most prominent is potassium hexafluorozironate, formed by fusion of potassium fluoride and zirconium tetrafluoride:
- ZrF
4 + 2 KF → K
2ZrF
6
Applications
The major and perhaps only commercial application of zirconium fluoride is as a precursor to ZBLAN glasses.[2]
Mixture of sodium fluoride, zirconium fluoride, and uranium tetrafluoride (53-41-6 mol.%) was used as a coolant in the Aircraft Reactor Experiment. A mixture of lithium fluoride, beryllium fluoride, zirconium fluoride, and uranium-233 tetrafluoride was used in the Molten-Salt Reactor Experiment. (Uranium-233 is used in the thorium fuel cycle reactors.)[citation needed]
References
- ↑ "Zirconium compounds (as Zr)". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/7440677.html.
- ↑ 2.0 2.1 2.2 Nielsen, Ralph (2000). "Zirconium and Zirconium Compounds". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a28_543. ISBN 3527306730.
- ↑ Paul L. Brown; Federico J. Mompean; Jane Perrone; Myriam Illemassène (2005). Chemical thermodynamics of zirconium. Gulf Professional Publishing. p. 144. ISBN 0-444-51803-7. https://books.google.com/books?id=DvqwTdVhjMEC&pg=PA144.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 965. ISBN 978-0-08-037941-8.
- ORNL/TM-2006/12 Assessment of Candidate Molten Salt Coolants for the Advanced High-Temperature Reactor (AHTR), March 2006 (Accessed 2008/9/18)
 |
Categories: [Fluorides] [Metal halides]
↧ Download as ZWI file | Last modified: 09/22/2023 20:29:20 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Zirconium_tetrafluoride | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF