Selenium Tetrafluoride
 From Handwiki
From Handwiki 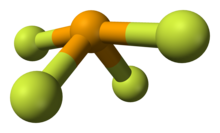
| |
| Identifiers | |
|---|---|
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
SeF4 |
| Molar mass | 154.954 g/mol |
| Appearance | colourless liquid |
| Density | 2.77 g/cm3 |
| Melting point | −13.2 °C (8.2 °F; 259.9 K) |
| Boiling point | 101 °C (214 °F; 374 K) |
| Hazards | |
| NFPA 704 (fire diamond) | 
0
3
2 |
| Related compounds | |
Other anions
|
selenium dioxide, selenium(IV) chloride, selenium(IV) bromide |
Other cations
|
sulfur tetrafluoride, tellurium(IV) fluoride |
Related compounds
|
selenium difluoride, selenium hexafluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.
Synthesis
The first reported synthesis of selenium tetrafluoride was by Paul Lebeau in 1907, who treated selenium with fluorine:[1]
- Se + 2 F2 → SeF4
A synthesis involving more easily handled reagents entails the fluorination of selenium dioxide with sulfur tetrafluoride:[2]
- SF4 + SeO2 → SeF4 + SO2
An intermediate in this reaction is seleninyl fluoride (SeOF2).
Other methods of preparation include fluorinating elemental selenium with chlorine trifluoride:
- 3 Se + 4 ClF3 → 3 SeF4 + 2 Cl2
Structure and bonding
Selenium in SeF4 has an oxidation state of +4. Its shape in the gaseous phase is similar to that of SF4, having a see-saw shape. VSEPR theory predicts a pseudo-trigonal pyramidal disposition of the five electron pairs around the selenium atom. The axial Se-F bonds are 177 pm with an F-Se-F bond angle of 169.2°. The two other fluorine atoms are attached by shorter bonds (168 pm), with an F-Se-F bond angle of 100.6°. In solution at low concentrations this monomeric structure predominates, but at higher concentrations evidence suggests weak association between SeF4 molecules leading to a distorted octahedral coordination around the selenium atom. In the solid the selenium center also has a distorted octahedral environment.
Reactions
In HF, SeF4 behaves as a weak base, weaker than sulfur tetrafluoride, SF4 (Kb= 2 X 10−2):
- SeF4 + HF → SeF3+ + HF2−; (Kb = 4 X 10−4)
Ionic adducts containing the SeF3+ cation are formed with SbF5, AsF5, NbF5, TaF5, and BF3.[3] With caesium fluoride, CsF, the SeF5− anion is formed, which has a square pyramidal structure similar to the isoelectronic chlorine pentafluoride, ClF5 and bromine pentafluoride, BrF5.[4] With 1,1,3,3,5,5-hexamethylpiperidinium fluoride or 1,2-dimethylpropyltrimethylammonium fluoride, the SeF62− anion is formed. This has a distorted octahedral shape which contrasts to the regular octahedral shape of the analogous SeCl62−. [5]
References
- ↑ Paul Lebeau (1907). "Action of Fluorine on Selenium Tetrafluoride of Selenium". Comptes Rendus de l'Académie des Sciences de Paris 144: 1042.
- ↑ Konrad Seppelt, Dieter Lentz, Gerhard Klöter "Selenium Tetrafluoride, Selenium Difluoride Oxide (Seleninyl Fluoride), and Xenon Bis[Pentafluorooxoselenate(VI)]" Inorg. Synth., 1987, vol. 24, 27-31. doi:10.1002/9780470132555.ch9
- ↑ R. J. Gillespie; A. Whitla (1970). "Selenium tetrafluoride adducts. II. Adducts with boron trifluoride and some pentafluorides". Can. J. Chem. 48 (4): 657–663. doi:10.1139/v70-106.
- ↑ K. O. Christe; E. C. Curtis; C. J. Schack; D. Pilipovich (1972). "Vibrational Spectra and Force Constants of the Square-Pyramidal Anions SF5−, SeF5−, and TeF5−". Inorganic Chemistry 11 (7): 1679–1682. doi:10.1021/ic50113a046.
- ↑ Ali Reza Mahjoub; Xiongzhi Zhang; Konrad Seppelt (1995). "Reactions of the Naked Fluoride Ion: Syntheses and Structures of SeF62− and BrF6−". Chemistry: A European Journal 1 (4): 261–265. doi:10.1002/chem.19950010410.
- Selenium: Inorganic Chemistry Krebs. B., Bonmann S., Eidenschink I.; Encyclopedia of Inorganic Chemistry (1994) John Wiley and Sons ISBN:0-471-93620-0
See also
- Selenium hexafluoride
- Sulfur tetrafluoride
- Tellurium tetrafluoride
External links
- WebBook page for SeF4
 |
Categories: [Fluorides] [Fluorinating agents]
↧ Download as ZWI file | Last modified: 04/23/2024 08:49:28 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Selenium_tetrafluoride | License: CC BY-SA 3.0

 KSF
KSF