Glycerol 2-Phosphate
 From Handwiki
From Handwiki 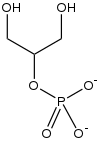
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,3-Dihydroxypropan-2-yl dihydrogen phosphate | |
| Other names
1,2,3-Propanetriol, 2-(dihydrogen phosphate)
Glycerol 2-phosphate β-Glycerophosphate β-Phosphoglycerol BGP | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| DrugBank |
|
| EC Number |
|
| KEGG |
|
| MeSH | Beta-glycerophosphoric+acid |
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C3H9O6P |
| Molar mass | 172.073 g·mol−1 |
| Appearance | forms colorless salts |
| Related compounds | |
Related organophosphates
|
Glycerol 1-phosphate Glycerol 3-phosphate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
Glycerol 2-phosphate is the conjugate base of phosphoric ester of glycerol. It is commonly known as β-glycerophosphate or BGP. Unlike glycerol 1-phosphate and glycerol 3-phosphate, this isomer is not chiral. It is also less common.
Applications
β-Glycerophosphate is an inhibitor of the enzyme serine-threonine phosphatase. It is often used in combination with other phosphatase/protease inhibitors for broad spectrum inhibition.[1][2]
β-Glycerophosphate is also used to drive osteogenic differentiation of bone marrow stem cells in vitro.[3]
β-Glycerophosphate is used to buffer M17 media for Lactococcus culture in recombinant protein expression.[4]
Notes
- ↑ "β-Glycerophosphate disodium salt hydrate". Sigma-Aldrich. https://www.sigmaaldrich.com/catalog/product/sigma/g6376. Retrieved 2018-05-30.
- ↑ "Protease and Phosphatase Inhibitors". Thermo Fisher Scientific. https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/protease-phosphatase-inhibitors.html. Retrieved 2018-05-30.
- ↑ Langenbach & Handschel (2013). "Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro". Stem Cell Research & Therapy 4 (5): 117. doi:10.1186/scrt328. PMID 24073831.
- ↑ Terzaghi & Sandine (1975). "Improved medium for lactic streptococci and their bacteriophages". Applied Microbiology 29 (6): 807–813. doi:10.1128/AM.29.6.807-813.1975. PMID 16350018.
 |
Categories: [Organophosphates]
↧ Download as ZWI file | Last modified: 05/09/2023 01:55:54 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Glycerol_2-phosphate | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF