Shingles
 From Mdwiki
From Mdwiki | Shingles | |
|---|---|
| Other names: Zoster, herpes zoster, zona | |
 | |
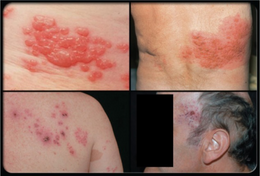
| Herpes zoster blisters on the neck and shoulder | |
| Specialty | Dermatology |
| Symptoms | Painful rash occurring in a stripe[1] |
| Complications | Postherpetic neuralgia[1] |
| Duration | 2–4 weeks[2] |
| Causes | Varicella zoster virus (VZV)[1] |
| Risk factors | Old age, poor immune function, having had chickenpox before 18 months of age[1] |
| Diagnostic method | Based on symptoms[3] |
| Differential diagnosis | Herpes simplex, angina, insect bites[4] |
| Prevention | Shingles vaccine[1] |
| Medication | Aciclovir (if given early), pain medication[3] |
| Frequency | 33% (at some point)[1] |
| Deaths | 6,400 (with chickenpox)[5] |
Shingles, also known as zoster or herpes zoster, is a viral disease characterized by a painful skin rash with blisters in a localized area.[2][6] Typically the rash occurs in a single, wide stripe either on the left or right side of the body or face.[1] Two to four days before the rash occurs there may be tingling or local pain in the area.[1][7] Otherwise there are typically few symptoms though some may have fever or headache, or feel tired.[1][8] The rash usually heals within two to four weeks;[2] however, some people develop ongoing nerve pain which can last for months or years, a condition called postherpetic neuralgia (PHN).[1] In those with poor immune function the rash may occur widely.[1] If the rash involves the eye, vision loss may occur.[2][9]
Shingles is due to a reactivation of varicella zoster virus (VZV) in a person's body.[1] The disease chickenpox is caused by the initial infection with VZV.[1] Once chickenpox has resolved, the virus may remain inactive in nerve cells.[1] When it reactivates, it travels from the nerve body to the endings in the skin, producing blisters.[7] Risk factors for reactivation include old age, poor immune function, and having had chickenpox before 18 months of age.[1] How the virus remains in the body or subsequently re-activates is not well understood.[1] Exposure to the virus in the blisters can cause chickenpox in someone who has not had it, but will not trigger shingles.[10] Diagnosis is typically based on a person's signs and symptoms.[3] Varicella zoster virus is not the same as herpes simplex virus; however, they belong to the same family of viruses.[11]
The shingles vaccine reduces the risk of shingles by 50% to 90%, depending on the vaccine used.[1][12] It also decreases rates of postherpetic neuralgia, and if shingles occurs, its severity.[1] If shingles develops, antiviral medications such as aciclovir can reduce the severity and duration of disease if started within 72 hours of the appearance of the rash.[3] Evidence does not show a significant effect of antivirals or steroids on rates of postherpetic neuralgia.[13][14] Paracetamol, NSAIDs, or opioids may be used to help with the acute pain.[3]
It is estimated that about a third of people develop shingles at some point in their life.[1] While more common among older people, children may also get the disease.[11] The number of new cases per year ranges from 1.2 to 3.4 per 1,000 person-years among healthy individuals to 3.9 to 11.8 per 1,000 person-years among those older than 65 years of age.[8] About half of those living to age 85 will have at least one attack, and less than 5% will have more than one attack.[1][15] The disease has been recognized since ancient times.[1]
Signs and symptoms[edit | edit source]

The earliest symptoms of shingles, which include headache, fever, and malaise, are nonspecific, and may result in an incorrect diagnosis.[8][16] These symptoms are commonly followed by sensations of burning pain, itching, hyperesthesia (oversensitivity), or paresthesia ("pins and needles": tingling, pricking, or numbness).[17] Pain can be mild to extreme in the affected dermatome, with sensations that are often described as stinging, tingling, aching, numbing or throbbing, and can be interspersed with quick stabs of agonizing pain.[18]
Shingles in children is often painless, but people are more likely to get shingles as they age, and the disease tends to be more severe.[19]
In most cases after one to two days, but sometimes as long as three weeks, the initial phase is followed by the appearance of the characteristic skin rash. The pain and rash most commonly occur on the torso, but can appear on the face, eyes or other parts of the body. At first the rash appears similar to the first appearance of hives; however, unlike hives, shingles causes skin changes limited to a dermatome, normally resulting in a stripe or belt-like pattern that is limited to one side of the body and does not cross the midline.[17] Zoster sine herpete ("zoster without herpes") describes a person who has all of the symptoms of shingles except this characteristic rash.[20]
Later the rash becomes vesicular, forming small blisters filled with a serous exudate, as the fever and general malaise continue. The painful vesicles eventually become cloudy or darkened as they fill with blood, and crust over within seven to ten days; usually the crusts fall off and the skin heals, but sometimes, after severe blistering, scarring and discolored skin remain.[17]
| Day 1 | Day 2 | Day 5 | Day 6 |
|---|---|---|---|
 |
 |
 |
 |
Face[edit | edit source]
Shingles may have additional symptoms, depending on the dermatome involved. The trigeminal nerve is the most commonly involved nerve,[21] of which the ophthalmic division is the most commonly involved branch.[22] When the virus is reactivated in this nerve branch it is termed zoster ophthalmicus. The skin of the forehead, upper eyelid and orbit of the eye may be involved. Zoster ophthalmicus occurs in approximately 10% to 25% of cases. In some people, symptoms may include conjunctivitis, keratitis, uveitis, and optic nerve palsies that can sometimes cause chronic ocular inflammation, loss of vision, and debilitating pain.[23]
Shingles oticus, also known as Ramsay Hunt syndrome type II, involves the ear. It is thought to result from the virus spreading from the facial nerve to the vestibulocochlear nerve. Symptoms include hearing loss and vertigo (rotational dizziness).[24]
Shingles may occur in the mouth if the maxillary or mandibular division of the trigeminal nerve is affected,[25] in which the rash may appear on the mucous membrane of the upper jaw (usually the palate, sometimes the gums of the upper teeth) or the lower jaw (tongue or gums of the lower teeth) respectively.[26] Oral involvement may occur alone or in combination with a rash on the skin over the cutaneous distribution of the same trigeminal branch.[25] As with shingles of the skin, the lesions tend to only involve one side, distinguishing it from other oral blistering conditions.[26] In the mouth, shingles appears initially as 1–4 mm opaque blisters (vesicles),[25] which break down quickly to leave ulcers that heal within 10–14 days.[26] The prodromal pain (before the rash) may be confused with toothache.[25] Sometimes this leads to unnecessary dental treatment.[26] Post herpetic neuralgia uncommonly is associated with shingles in the mouth.[26] Unusual complications may occur with intra-oral shingles that are not seen elsewhere. Due to the close relationship of blood vessels to nerves, the virus can spread to involve the blood vessels and compromise the blood supply, sometimes causing ischemic necrosis.[25] Therefore, oral involvement rarely causes complications, such as osteonecrosis, tooth loss, periodontitis (gum disease), pulp calcification, pulp necrosis, periapical lesions and tooth developmental anomalies.[21]
-
Herpes zoster
-
Herpes zoster
-
Herpes zoster
-
Herpes zoster
Disseminated shingles[edit | edit source]
In those with poor immune function, disseminated shingles may occur (wide rash).[1] It is defined as more than twenty skin lesions appearing outside either the primarily affected dermatome or dermatomes directly adjacent to it. Besides the skin, other organs, such as the liver or brain, may also be affected (causing hepatitis or encephalitis,[27][28] respectively), making the condition potentially lethal.[29]: 380
Pathophysiology[edit | edit source]
_Virus_PHIL_1878_lores.jpg)

The causative agent for shingles is the varicella zoster virus (VZV) – a double-stranded DNA virus related to the herpes simplex virus. Most individuals are infected with this virus as children which causes an episode of chickenpox. The immune system eventually eliminates the virus from most locations, but it remains dormant (or latent) in the ganglia adjacent to the spinal cord (called the dorsal root ganglion) or the trigeminal ganglion in the base of the skull.[31]
Shingles occurs only in people who have been previously infected with VZV; although it can occur at any age, approximately half of the cases in the United States occur in those aged 50 years or older.[32] Repeated attacks of shingles are rare,[17] and it is extremely rare for a person to have more than three recurrences.[31]
The disease results from virus particles in a single sensory ganglion switching from their latent lysogenic cycles to their active lytic cycles.[33] In contrast to the herpes simplex virus, the latency of VZV is poorly understood. The virus has never been successfully recovered from human nerve cells by cell culture. Virus-specific proteins continue to be made by the infected cells during the latent period, so true latency, as opposed to chronic, low-level, active infection, has not been proven to occur in VZV infections.[34][35] Although VZV has been detected in autopsies of nervous tissue,[36] there are no methods to find dormant virus in the ganglia of living people.
Unless the immune system is compromised, it suppresses reactivation of the virus and prevents shingles outbreaks. Why this suppression sometimes fails is poorly understood,[37] but shingles is more likely to occur in people whose immune systems are impaired due to aging, immunosuppressive therapy, psychological stress, or other factors.[38][39] Upon reactivation, the virus replicates in neuronal cell bodies, and virions are shed from the cells and carried down the axons to the area of skin innervated by that ganglion. In the skin, the virus causes local inflammation and blistering. The short- and long-term pain caused by shingles outbreaks originates from inflammation of affected nerves due to the widespread growth of the virus in those areas.[40]
As with chickenpox and other forms of alpha-herpesvirus infection, direct contact with an active rash can spread the virus to a person who lacks immunity to it. This newly infected individual may then develop chickenpox, but will not immediately develop shingles.[17]
The complete sequence of the viral genome was published in 1986.[41]
Diagnosis[edit | edit source]
If the rash has appeared, identifying this disease (making a differential diagnosis) requires only a visual examination, since very few diseases produce a rash in a dermatomal pattern (see map). However, herpes simplex virus (HSV) can occasionally produce a rash in such a pattern (zosteriform herpes simplex).[42][43] The Tzanck smear is helpful for diagnosing acute infection with a herpes virus, but does not distinguish between HSV and VZV.[44]
When the rash is absent (early or late in the disease, or in the case of zoster sine herpete), shingles can be difficult to diagnose.[45] Apart from the rash, most symptoms can occur also in other conditions.
Laboratory tests are available to diagnose shingles. The most popular test detects VZV-specific IgM antibody in blood; this appears only during chickenpox or shingles and not while the virus is dormant.[46] In larger laboratories, lymph collected from a blister is tested by polymerase chain reaction for VZV DNA, or examined with an electron microscope for virus particles.[47] Molecular biology tests based on in vitro nucleic acid amplification (PCR tests) are currently considered the most reliable. Nested PCR test has high sensitivity, but is susceptible to contamination leading to false positive results. The latest real-time PCR tests are rapid, easy to perform, and as sensitive as nested PCR, and have a lower risk of contamination. They also have more sensitivity than viral cultures.[48]
-
Images of Herpes zoster (shingles) rash
-
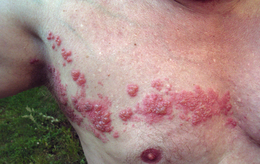
Shingles on the chest
Differential diagnosis[edit | edit source]
Shingles can be confused with herpes simplex, dermatitis herpetiformis and impetigo, and skin reactions caused by contact dermatitis, candidiasis, certain drugs and insect bites.[49]
Prevention[edit | edit source]
Shingles can be prevented by the chicken pox vaccine if the vaccine is administered before the individual gets chickenpox.[50] If primary infection has already occurred, there are shingles vaccines that reduce the risk of developing shingles or developing severe shingles if the disease occurs.[1][12] They include a live attenuated virus vaccine, Zostavax, and an adjuvanted subunit vaccine, Shingrix.[51][52][53]
A review by Cochrane concluded that Zostavax was useful for preventing shingles for at least three years.[7] This equates to about 50% relative risk reduction. The vaccine reduced rates of persistent, severe pain after shingles by 66% in people who contracted shingles despite vaccination.[54] Vaccine efficacy was maintained through four years of follow up.[54] It has been recommended that people with primary or acquired immunodeficiency should not receive the live vaccine.[54]
Two doses of Shingrix are recommended, which provide about 90% protection at 3.5 years.[53][51] As of 2016, it had been studied only in people with an intact immune system.[12] It appears to also be effective in the very old.[12]
In England, Zostavax is offered by the National Health Service (NHS) to all people at age 70 and 78.[55] By August 2017, just under half of eligible 70–78 year olds had been vaccinated.[56] About 3% of those eligible by age but who have conditions that suppress their immune system, should not receive the vaccine.[57] There had been 1,104 adverse reaction reports by April 2018.[57] In the US, it is recommended that healthy adults 50 years and older receive two doses of Shingrix, two to six months apart.[51][58]
Treatment[edit | edit source]
The aims of treatment are to limit the severity and duration of pain, shorten the duration of a shingles episode, and reduce complications. Symptomatic treatment is often needed for the complication of postherpetic neuralgia.[59] However, a study on untreated shingles shows that, once the rash has cleared, postherpetic neuralgia is very rare in people under 50 and wears off in time; in older people the pain wore off more slowly, but even in people over 70, 85% were free from pain a year after their shingles outbreak.[60]
Analgesics[edit | edit source]
People with mild to moderate pain can be treated with over-the-counter pain medications. Topical lotions containing calamine can be used on the rash or blisters and may be soothing. Occasionally, severe pain may require an opioid medication, such as morphine. Once the lesions have crusted over, capsaicin cream (Zostrix) can be used. Topical lidocaine and nerve blocks may also reduce pain.[61] Administering gabapentin along with antivirals may offer relief of postherpetic neuralgia.[59]
Antivirals[edit | edit source]
Antiviral drugs may reduce the severity and duration of shingles;[62] however, they do not prevent postherpetic neuralgia.[63] Of these drugs, aciclovir has been the standard treatment, but the newer drugs valaciclovir and famciclovir demonstrate similar or superior efficacy and good safety and tolerability.[59] The drugs are used both for prevention (for example in people with HIV/AIDS) and as therapy during the acute phase. Complications in immunocompromised individuals with shingles may be reduced with intravenous aciclovir. In people who are at a high risk for repeated attacks of shingles, five daily oral doses of aciclovir are usually effective.[24]
Steroids[edit | edit source]
Corticosteroids do not appear to decrease the risk of long-term pain.[14] Side effects however appear to be minimal. Their use in Ramsay Hunt syndrome had not been properly studied as of 2008.[64]
Zoster ophthalmicus[edit | edit source]

Treatment for zoster ophthalmicus is similar to standard treatment for shingles at other sites. A recent trial comparing acyclovir with its prodrug, valacyclovir, demonstrated similar efficacies in treating this form of the disease.[65] The significant advantage of valacyclovir over acyclovir is its dosing of only three times/day (compared with acyclovir's five times/day dosing), which could make it more convenient for people and improve adherence with therapy.[66]
Prognosis[edit | edit source]
The rash and pain usually subside within three to five weeks, but about one in five people develops a painful condition called postherpetic neuralgia, which is often difficult to manage. In some people, shingles can reactivate presenting as zoster sine herpete: pain radiating along the path of a single spinal nerve (a dermatomal distribution), but without an accompanying rash. This condition may involve complications that affect several levels of the nervous system and cause many cranial neuropathies, polyneuritis, myelitis, or aseptic meningitis. Other serious effects that may occur in some cases include partial facial paralysis (usually temporary), ear damage, or encephalitis.[24] Although initial infections with VZV during pregnancy, causing chickenpox, may lead to infection of the fetus and complications in the newborn, chronic infection or reactivation in shingles are not associated with fetal infection.[67][68]
There is a slightly increased risk of developing cancer after a shingles episode. However, the mechanism is unclear and mortality from cancer did not appear to increase as a direct result of the presence of the virus.[69] Instead, the increased risk may result from the immune suppression that allows the reactivation of the virus.[70]
Although shingles typically resolves within 3–5 weeks, certain complications may arise:
- Secondary bacterial infection.[9]
- Motor involvement,[9] including weakness especially in "motor herpes zoster".[71]
- Eye involvement: trigeminal nerve involvement (as seen in herpes ophthalmicus) should be treated early and aggressively as it may lead to blindness. Involvement of the tip of the nose in the zoster rash is a strong predictor of herpes ophthalmicus.[72]
- Postherpetic neuralgia, a condition of chronic pain following shingles.
Epidemiology[edit | edit source]
Varicella zoster virus (VZV) has a high level of infectivity and has a worldwide prevalence.[73] Shingles is a re-activation of latent VZV infection: zoster can only occur in someone who has previously had chickenpox (varicella).
Shingles has no relationship to season and does not occur in epidemics. There is, however, a strong relationship with increasing age.[19][38] The incidence rate of shingles ranges from 1.2 to 3.4 per 1,000 person‐years among younger healthy individuals, increasing to 3.9–11.8 per 1,000 person‐years among those older than 65 years,[8][19] and incidence rates worldwide are similar.[8][74] This relationship with age has been demonstrated in many countries,[8][74][75][76][77][78] and is attributed to the fact that cellular immunity declines as people grow older.
Another important risk factor is immunosuppression.[79][80][81] Other risk factors include psychological stress.[18][82][83] According to a study in North Carolina, "black subjects were significantly less likely to develop zoster than were white subjects."[84][85] It is unclear whether the risk is different by sex. Other potential risk factors include mechanical trauma and exposure to immunotoxins.[38][83]
There is no strong evidence for a genetic link or a link to family history. A 2008 study showed that people with close relatives who had shingles were twice as likely to develop it themselves,[86] but a 2010 study found no such link.[83]
Adults with latent VZV infection who are exposed intermittently to children with chickenpox receive an immune boost.[19][83] This periodic boost to the immune system helps to prevent shingles in older adults. When routine chickenpox vaccination was introduced in the United States, there was concern that, because older adults would no longer receive this natural periodic boost, there would be an increase in the incidence of shingles.
Multiple studies and surveillance data, at least when viewed superficially, demonstrate no consistent trends in incidence in the U.S. since the chickenpox vaccination program began in 1995.[87] However, upon closer inspection, the two studies that showed no increase in shingles incidence were conducted among populations where varicella vaccination was not as yet widespread in the community.[88][89] A later study by Patel et al. concluded that since the introduction of the chickenpox vaccine, hospitalization costs for complications of shingles increased by more than $700 million annually for those over age 60.[90] Another study by Yih et al. reported that as varicella vaccine coverage in children increased, the incidence of varicella decreased, and the occurrence of shingles among adults increased by 90%.[91] The results of a further study by Yawn et al. showed a 28% increase in shingles incidence from 1996 to 2001.[92] It is likely that incidence rate will change in the future, due to the aging of the population, changes in therapy for malignant and autoimmune diseases, and changes in chickenpox vaccination rates; a wide adoption of zoster vaccination could dramatically reduce the incidence rate.[8]
In one study, it was estimated that 26% of those who contract shingles eventually present complications. Postherpetic neuralgia arises in approximately 20% of people with shingles.[93] A study of 1994 California data found hospitalization rates of 2.1 per 100,000 person-years, rising to 9.3 per 100,000 person-years for ages 60 and up.[94] An earlier Connecticut study found a higher hospitalization rate; the difference may be due to the prevalence of HIV in the earlier study, or to the introduction of antivirals in California before 1994.[95]
History[edit | edit source]
Shingles has a long recorded history, although historical accounts fail to distinguish the blistering caused by VZV and those caused by smallpox,[32] ergotism, and erysipelas. In the late 18th century William Heberden established a way to differentiate between shingles and smallpox,[96] and in the late 19th century shingles was differentiated from erysipelas. In 1831 Richard Bright hypothesized that the disease arose from the dorsal root ganglion, and an 1861 paper by Felix von Bärensprung confirmed this.[97]
The first indications that chickenpox and shingles were caused by the same virus were noticed at the beginning of the 20th century. Physicians began to report that cases of shingles were often followed by chickenpox in the younger people who lived with the person with shingles. The idea of an association between the two diseases gained strength when it was shown that lymph from a person with shingles could induce chickenpox in young volunteers. This was finally proved by the first isolation of the virus in cell cultures, by the Nobel laureate Thomas Huckle Weller, in 1953.[98]
Until the 1940s the disease was considered benign, and serious complications were thought to be very rare.[99] However, by 1942, it was recognized that shingles was a more serious disease in adults than in children and that it increased in frequency with advancing age. Further studies during the 1950s on immunosuppressed individuals showed that the disease was not as benign as once thought, and the search for various therapeutic and preventive measures began.[100] By the mid-1960s, several studies identified the gradual reduction in cellular immunity in old age, observing that in a cohort of 1,000 people who lived to the age of 85, approximately 500 (i.e., 50%) would have at least one attack of shingles, and 10 (i.e., 1%) would have at least two attacks.[101]
In historical shingles studies, shingles incidence generally increased with age. However, in his 1965 paper, Hope-Simpson suggested that the "peculiar age distribution of zoster may in part reflect the frequency with which the different age groups encounter cases of varicella and because of the ensuing boost to their antibody protection have their attacks of zoster postponed".[19] Lending support to this hypothesis that contact with children with chickenpox boosts adult cell-mediated immunity to help postpone or suppress shingles, a study by Thomas et al. reported that adults in households with children had lower rates of shingles than households without children.[102] Also, the study by Terada et al. indicated that pediatricians reflected incidence rates from 1/2 to 1/8 that of the general population their age.[103]
Etymology[edit | edit source]
The family name of all the herpesviruses derives from the Greek word herpēs,[104] from herpein ("to creep"),[105][106][107] referring to the latent, recurring infections typical of this group of viruses. Zoster comes from Greek zōstēr,[108] meaning "belt" or "girdle", after the characteristic belt-like dermatomal rash.[109] The common name for the disease, shingles, derives from the Latin cingulus, a variant of Latin cingulum[110] meaning "girdle".[111]
In Arabic its name means "belt of fire", while in Spanish it means "small snake"; in Hindi it means "big rash"[112] and in Norwegian its name is helvetesild, literally "hell's fire".[113]
Research[edit | edit source]
Until the mid 1990s, infectious complications of the central nervous system (CNS) caused by VZV reactivation were regarded as rare. The presence of rash, as well as specific neurological symptoms, were required to diagnose a CNS infection caused by VZV. Since 2000, PCR testing has become more widely used, and the number of diagnosed cases of CNS infection has increased.[114]
Classic textbook descriptions state that VZV reactivation in the CNS is restricted to immunocompromised individuals and the elderly; however, recent studies have found that most patients are immunocompetent, and less than 60 years old. Old references cite vesicular rash as a characteristic finding; however, recent studies have found that rash is only present in 45% of cases.[114] In addition, systemic inflammation is not as reliable an indicator as previously thought: the mean level of C-reactive protein and mean white blood cell count are within the normal range in patients with VZV meningitis.[115] MRI and CT scans are usually normal in cases of VZV reactivation in the CNS. CSF pleocytosis, previously thought to be a strong indicator of VZV encephalitis, was absent in half of a group of patients diagnosed with VZV encephalitis by PCR.[114]
The frequency of CNS infections presented at the emergency room of a community hospital is not negligible, so a means of diagnosing cases is needed. PCR is not a foolproof method of diagnosis, but because so many other indicators have turned out to not be reliable in diagnosing VZV infections in the CNS, screening for VZV by PCR is recommended. Negative PCR does not rule out VZV involvement, but a positive PCR can be used for diagnosis, and appropriate treatment started (for example, antivirals can be prescribed rather than antibiotics).[114]
The introduction of DNA analysis techniques has shown some complications of varicella-zoster to be more common than previously thought. For example, sporadic meningoencephalitis (ME) caused by varicella-zoster was regarded as rare disease, mostly related to childhood chickenpox. However, meningoencephalitis caused by varicella-zoster is increasingly recognized as a predominant cause of ME among immunocompetent adults in non-epidemic circumstances.[116]
Diagnosis of complications of varicella-zoster, particularly in cases where the disease reactivates after years or decades of latency, is difficult. A rash (shingles) can be present or absent. Symptoms vary, and there is significant overlap in symptoms with herpes-simplex symptoms.[116]
Although DNA analysis techniques such as polymerase chain reaction (PCR) can be used to look for DNA of herpesviruses in spinal fluid or blood, the results may be negative, even in cases where other definitive symptoms exist.[117] Notwithstanding these limitations, the use of PCR has resulted in an advance in the state of the art in our understanding of herpesviruses, including VZV, during the 1990s and 2000s. For example, in the past, clinicians believed that encephalitis was caused by herpes simplex, and that patients always died or developed serious long term function problems. People were diagnosed at autopsy or by brain biopsy. Brain biopsy is not undertaken lightly: it is reserved only for serious cases that cannot be diagnosed by less invasive methods. For this reason, knowledge of these herpes virus conditions was limited to severe cases. DNA techniques have made it possible to diagnose "mild" cases, caused by VZV or HSV, in which the symptoms include fever, headache, and altered mental status. Mortality rates in treated patients are decreasing.[116]
References[edit | edit source]
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 Hamborsky J, Kroger A, Wolfe S, eds. (2015). "Chapter 22: Varicella". Epidemiology and Prevention of Vaccine-Preventable Diseases (13th ed.). Washington D.C.: U.S. Centers for Disease Control and Prevention (CDC). ISBN 978-0990449119. Archived from the original on 2016-12-30. Retrieved 2020-01-09.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 2.0 2.1 2.2 2.3 "Shingles (Herpes Zoster) Signs & Symptoms". Centers for Disease Control and Prevention (CDC). May 1, 2014. Archived from the original on 26 May 2015. Retrieved 26 May 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 3.0 3.1 3.2 3.3 3.4 Cohen, JI (18 July 2013). "Clinical practice: Herpes zoster". The New England Journal of Medicine. 369 (3): 255–63. doi:10.1056/NEJMcp1302674. PMC 4789101. PMID 23863052.
- ↑ Kahan, Scott (2003). In a Page Medicine. Lippincott Williams & Wilkins. p. 215. ISBN 9781405103251. Archived from the original on 2019-12-17. Retrieved 2017-09-11.
- ↑ GBD 2015 Mortality and Causes of Death Collaborators (8 October 2016). "Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015". Lancet. 388 (10053): 1459–1544. doi:10.1016/s0140-6736(16)31012-1. PMC 5388903. PMID 27733281.
- ↑ Rajendran, Arya; Sivapathasundharam, B (2014). Shafer's textbook of oral pathology (Seventh ed.). p. 351. ISBN 9788131238004. Archived from the original on 2019-12-17. Retrieved 2017-09-11.
- ↑ 7.0 7.1 7.2 Gagliardi, Anna Mz; Andriolo, Brenda Ng; Torloni, Maria Regina; Soares, Bernardo Go; de Oliveira Gomes, Juliana; Andriolo, Regis B.; Canteiro Cruz, Eduardo (7 November 2019). "Vaccines for preventing herpes zoster in older adults". The Cochrane Database of Systematic Reviews. 2019 (11). doi:10.1002/14651858.CD008858.pub4. ISSN 1469-493X. PMC 6836378. PMID 31696946.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Dworkin RH, Johnson RW, Breuer J, et al. (2007). "Recommendations for the management of herpes zoster". Clin. Infect. Dis. 44 Suppl 1: S1–26. doi:10.1086/510206. PMID 17143845.
- ↑ 9.0 9.1 9.2 Johnson RW, Alvarez-Pasquin MJ, Bijl M, et al. (2015). "Herpes zoster epidemiology, management, and disease and economic burden in Europe: A multidisciplinary perspective". Therapeutic Advances in Vaccines. 3 (4): 109–20. doi:10.1177/2051013615599151. PMC 4591524. PMID 26478818.
- ↑ "Shingles (Herpes Zoster) Transmission". Centers for Disease Control and Prevention (CDC). September 17, 2014. Archived from the original on 6 May 2015. Retrieved 26 May 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 11.0 11.1 "Overview". Centers for Disease Control and Prevention (CDC). September 17, 2014. Archived from the original on 16 May 2015. Retrieved 26 May 2015.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 12.0 12.1 12.2 12.3 Cunningham, AL (2016). "The herpes zoster subunit vaccine". Expert Opinion on Biological Therapy. 16 (2): 265–71. doi:10.1517/14712598.2016.1134481. PMID 26865048.
- ↑ Chen N, Li Q, Yang J, et al. (6 February 2014). "Antiviral treatment for preventing postherpetic neuralgia". Cochrane Database of Systematic Reviews. 2 (2): CD006866. doi:10.1002/14651858.CD006866.pub3. PMID 24500927.
- ↑ 14.0 14.1 Han Y, Zhang J, Chen N, et al. (28 March 2013). Han Y (ed.). "Corticosteroids for preventing postherpetic neuralgia". Cochrane Database of Systematic Reviews. 3 (3): CD005582. doi:10.1002/14651858.CD005582.pub4. PMID 23543541.
- ↑ Honorio T. Benzon (2011). Essentials of Pain Medicine (3rd ed.). London: Elsevier Health Sciences. p. 358. ISBN 9781437735932. Archived from the original on 2019-12-17. Retrieved 2017-09-11.
- ↑ Zamula E (May–June 2001). "Shingles: an unwelcome encore". FDA Consumer. 35 (3): 21–25. PMID 11458545. Archived from the original on 2009-11-03. Retrieved 2010-01-05. Revised June 2005.
- ↑ 17.0 17.1 17.2 17.3 17.4 Stankus SJ, Dlugopolski M, Packer D (2000). "Management of herpes zoster (shingles) and postherpetic neuralgia". Am. Fam. Physician. 61 (8): 2437–44, 2447–48. PMID 10794584. Archived from the original on 2007-09-29.
- ↑ 18.0 18.1 Katz J, Cooper EM, Walther RR, et al. (2004). "Acute pain in herpes zoster and its impact on health-related quality of life". Clin. Infect. Dis. 39 (3): 342–48. doi:10.1086/421942. PMID 15307000.
- ↑ 19.0 19.1 19.2 19.3 19.4 Hope-Simpson RE (1965). "The nature of herpes zoster: a long-term study and a new hypothesis". Proceedings of the Royal Society of Medicine. 58 (1): 9–20. doi:10.1177/003591576505800106. PMC 1898279. PMID 14267505.
- ↑ Furuta Y, Ohtani F, Mesuda Y, et al. (2000). "Early diagnosis of zoster sine herpete and antiviral therapy for the treatment of facial palsy". Neurology. 55 (5): 708–10. doi:10.1212/WNL.55.5.708. PMID 10980741.
- ↑ 21.0 21.1 Gupta S, Sreenivasan V, Patil PB (2015). "Dental complications of herpes zoster: Two case reports and review of literature". Indian Journal of Dental Research. 26 (2): 214–19. doi:10.4103/0970-9290.159175. PMID 26096121.
- ↑ Samaranayake L (2 September 2011). Essential Microbiology for Dentistry (4th ed.). Elsevier Health Sciences. pp. 638–42. ISBN 978-0-7020-4695-7. Archived from the original on 8 September 2017.
- ↑ Shaikh S, Ta CN (2002). "Evaluation and management of herpes zoster ophthalmicus". Am. Fam. Physician. 66 (9): 1723–30. PMID 12449270. Archived from the original on 2008-05-14.
- ↑ 24.0 24.1 24.2 Johnson RW, Dworkin RH (2003). "Clinical review: Treatment of herpes zoster and postherpetic neuralgia". BMJ. 326 (7392): 748–50. doi:10.1136/bmj.326.7392.748. PMC 1125653. PMID 12676845. Archived from the original on 2008-01-31.
- ↑ 25.0 25.1 25.2 25.3 25.4 Chi, AC; Damm, DD; Neville, BW; Allen, CM; Bouquot, J (11 June 2008). Oral and Maxillofacial Pathology. Elsevier Health Sciences. pp. 250–53. ISBN 978-1-4377-2197-3. Archived from the original on 8 September 2017.
- ↑ 26.0 26.1 26.2 26.3 26.4 Glick M (1 September 2014). Burket's oral medicine (12th ed.). coco. pp. 62–65. ISBN 978-1-60795-188-9.
{{cite book}}: CS1 maint: url-status (link) - ↑ Chai W, Ho MG-R. Disseminated varicella zoster virus encephalitis. Lancet Available online 3 July 2014(0). Disseminated varicella zoster virus encephalitis : The Lancet at the Wayback Machine (archived 29 November 2014)
- ↑ Grahn A, Studahl M (September 2015). "Varicella-zoster virus infections of the central nervous system – Prognosis, diagnostics and treatment". Journal of Infection. 71 (3): 281–93. doi:10.1016/j.jinf.2015.06.004. PMID 26073188.
- ↑ James, William D.; Berger, Timothy G.; et al. (2006). Andrews' Diseases of the Skin: Clinical Dermatology. Saunders Elsevier. ISBN 978-0-7216-2921-6.
- ↑ Public Health Agency of Canada (30 April 2012), Pathogen Safety Data Sheets: Infectious Substances – Varicella-zoster virus, archived from the original on 22 March 2020, retrieved 22 March 2020
- ↑ 31.0 31.1 Steiner I, Kennedy PG, Pachner AR (2007). "The neurotropic herpes viruses: herpes simplex and varicella-zoster". Lancet Neurol. 6 (11): 1015–28. doi:10.1016/S1474-4422(07)70267-3. PMID 17945155.
- ↑ 32.0 32.1 Weinberg JM (2007). "Herpes zoster: epidemiology, natural history, and common complications". J. Am. Acad. Dermatol. 57 (6 Suppl): S130–35. doi:10.1016/j.jaad.2007.08.046. PMID 18021864.
- ↑ Gilden DH, Cohrs RJ, Mahalingam R (2003). "Clinical and molecular pathogenesis of varicella virus infection". Viral Immunol. 16 (3): 243–58. doi:10.1089/088282403322396073. PMID 14583142.
- ↑ Kennedy PG (2002). "Varicella-zoster virus latency in human ganglia". Rev. Med. Virol. 12 (5): 327–34. doi:10.1002/rmv.362. PMID 12211045.
- ↑ Kennedy PG (2002). "Key issues in varicella-zoster virus latency". J. Neurovirol. 8 Suppl 2 (2): 80–84. CiteSeerX 10.1.1.415.2755. doi:10.1080/13550280290101058. PMID 12491156.
- ↑ Mitchell BM, Bloom DC, Cohrs RJ, et al. (2003). "Herpes simplex virus-1 and varicella-zoster virus latency in ganglia" (PDF). J. Neurovirol. 9 (2): 194–204. doi:10.1080/13550280390194000. PMID 12707850. Archived (PDF) from the original on 2008-05-17.
- ↑ Donahue JG, Choo PW, Manson JE, et al. (1995). "The incidence of herpes zoster". Archives of Internal Medicine. 155 (15): 1605–09. doi:10.1001/archinte.155.15.1605. PMID 7618983.
- ↑ 38.0 38.1 38.2 Thomas SL, Hall AJ (2004). "What does epidemiology tell us about risk factors for herpes zoster?". Lancet Infect. Dis. 4 (1): 26–33. doi:10.1016/S1473-3099(03)00857-0. PMID 14720565.
- ↑ "Shingles". NHS.UK. Archived from the original on 26 September 2017. Retrieved 25 September 2017.
- ↑ Schmader K (2007). "Herpes zoster and postherpetic neuralgia in older adults". Clin. Geriatr. Med. 23 (3): 615–32, vii–viii. doi:10.1016/j.cger.2007.03.003. PMC 4859150. PMID 17631237.
- ↑ Davison, AJ, Scott, et al. (1986). "The complete DNA sequence of varicella-zoster virus". J. Gen. Virol. 67 (9): 1759–1816. doi:10.1099/0022-1317-67-9-1759. PMID 3018124.
- ↑ Koh MJ, Seah PP, Teo RY (Feb 2008). "Zosteriform herpes simplex" (PDF). Singapore Med. J. 49 (2): e59–60. PMID 18301829. Archived (PDF) from the original on 2014-06-02.
- ↑ Kalman CM, Laskin OL (Nov 1986). "Herpes zoster and zosteriform herpes simplex virus infections in immunocompetent adults". Am. J. Med. 81 (5): 775–78. doi:10.1016/0002-9343(86)90343-8. PMID 3022586.
- ↑ Oranje AP, Folkers E (1988). "The Tzanck smear: old, but still of inestimable value". Pediatr. Dermatol. 5 (2): 127–29. doi:10.1111/j.1525-1470.1988.tb01154.x. PMID 2842739.
- ↑ Chan J, Bergstrom RT, Lanza DC, et al. (2004). "Lateral sinus thrombosis associated with zoster sine herpete". Am. J. Otolaryngol. 25 (5): 357–60. doi:10.1016/j.amjoto.2004.03.007. PMID 15334402.
- ↑ Arvin AM (1996). "Varicella-zoster virus" (PDF). Clin. Microbiol. Rev. 9 (3): 361–81. doi:10.1128/CMR.9.3.361. PMC 172899. PMID 8809466. Archived (PDF) from the original on 2008-06-25.
- ↑ Beards G, Graham C, Pillay D (1998). "Investigation of vesicular rashes for HSV and VZV by PCR". J. Med. Virol. 54 (3): 155–57. doi:10.1002/(SICI)1096-9071(199803)54:3<155::AID-JMV1>3.0.CO;2-4. PMID 9515761.
- ↑ De Paschale M, Clerici P (2016). "Microbiology laboratory and the management of mother-child varicella-zoster virus infection". World J Virol (Review). 5 (3): 97–124. doi:10.5501/wjv.v5.i3.97. PMC 4981827. PMID 27563537.
- ↑ Sampathkumar P, Drage LA, Martin DP (2009). "Herpes zoster (shingles) and postherpetic neuralgia". Mayo Clin Proc (Review). 84 (3): 274–80. doi:10.4065/84.3.274. PMC 2664599. PMID 19252116.
- ↑ Weinmann S, Naleway AL, Koppolu P, et al. (July 2019). "Incidence of Herpes Zoster Among Children: 2003-2014". Pediatrics. 144 (1): e20182917. doi:10.1542/peds.2018-2917. PMID 31182552.
- Lay summary in: "Two-for-One: Chickenpox Vaccine Lowers Shingles Risk in Children". Scientific American. 11 June 2019.
- ↑ 51.0 51.1 51.2 Dooling KL, Guo A, Patel M, et al. (January 2018). "Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines" (PDF). MMWR Morb. Mortal. Wkly. Rep. 67 (3): 103–108. doi:10.15585/mmwr.mm6703a5. PMC 5812314. PMID 29370152. Archived (PDF) from the original on 2021-08-29. Retrieved 2020-01-09.
- ↑ Harpaz R, Ortega-Sanchez IR, Seward JF (June 6, 2008). "Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm. Rep. 57 (RR–5): 1–30, quiz CE2–4. PMID 18528318. Archived from the original on November 17, 2009. Retrieved 2010-01-04.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 53.0 53.1 Cunningham AL, Lal H, Kovac M, et al. (September 2016). "Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older". N. Engl. J. Med. 375 (11): 1019–32. doi:10.1056/NEJMoa1603800. PMID 27626517.
- ↑ 54.0 54.1 54.2 Shapiro M, Kvern B, Watson P, et al. (October 2011). "Update on herpes zoster vaccination: a family practitioner's guide". Can. Fam. Physician. 57 (10): 1127–31. PMC 3192074. PMID 21998225.
- ↑ "The shingles immunisation programme: evaluation of the programme and implementation in 2018" (PDF). Public Health England (PHE). 9 April 2018. Archived (PDF) from the original on 9 January 2020. Retrieved 9 January 2020.
- ↑ "Herpes zoster (shingles) immunisation programme 2016 to 2017: evaluation report". GOV.UK. 15 December 2017. Archived from the original on 9 January 2020. Retrieved 9 January 2020.
- ↑ 57.0 57.1 "NHS England warning as vaccine programme extended". Health Service Journal. 18 April 2018. Archived from the original on 15 November 2019. Retrieved 10 June 2018.
- ↑ "Shingles (Herpes Zoster) Vaccination". Centers for Disease Control and Prevention (CDC). October 25, 2018. Archived from the original on 16 January 2020. Retrieved 18 January 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ 59.0 59.1 59.2 Tyring SK (2007). "Management of herpes zoster and postherpetic neuralgia". J. Am. Acad. Dermatol. 57 (6 Suppl): S136–42. doi:10.1016/j.jaad.2007.09.016. PMID 18021865.
- ↑ Helgason S, Petursson G, Gudmundsson S, et al. (2000). "Prevalence of postherpetic neuralgia after a single episode of herpes zoster: prospective study with long term follow up" (PDF). British Medical Journal. 321 (7264): 794–96. doi:10.1136/bmj.321.7264.794. PMC 27491. PMID 11009518. Archived from the original on 2009-02-09.
- ↑ Baron R (2004). "Post-herpetic neuralgia case study: optimizing pain control". Eur. J. Neurol. 11 Suppl 1: 3–11. doi:10.1111/j.1471-0552.2004.00794.x. PMID 15061819.
- ↑ Bader MS (Sep 2013). "Herpes zoster: diagnostic, therapeutic, and preventive approaches". Postgraduate Medicine. 125 (5): 78–91. doi:10.3810/pgm.2013.09.2703. PMID 24113666.
- ↑ Chen N, Li Q, Yang J, et al. (2014). He L (ed.). "Antiviral treatment for preventing postherpetic neuralgia". Cochrane Database of Systematic Reviews. 2 (2): CD006866. doi:10.1002/14651858.CD006866.pub3. PMID 24500927.
- ↑ Uscategui T, Doree C, Chamberlain IJ, et al. (Jul 16, 2008). "Corticosteroids as adjuvant to antiviral treatment in Ramsay Hunt syndrome (herpes zoster oticus with facial palsy) in adults". Cochrane Database of Systematic Reviews (3): CD006852. doi:10.1002/14651858.CD006852.pub2. PMID 18646170.
- ↑ Colin J, Prisant O, Cochener B, et al. (2000). "Comparison of the Efficacy and Safety of Valaciclovir and Acyclovir for the Treatment of Herpes zoster Ophthalmicus". Ophthalmology. 107 (8): 1507–11. doi:10.1016/S0161-6420(00)00222-0. PMID 10919899.
- ↑ Osterberg L, Blaschke T (2005). "Adherence to medication". The New England Journal of Medicine. 353 (5): 487–97. doi:10.1056/NEJMra050100. PMID 16079372.
- ↑ Paryani SG, Arvin AM (1986). "Intrauterine infection with varicella-zoster virus after maternal varicella". The New England Journal of Medicine. 314 (24): 1542–46. doi:10.1056/NEJM198606123142403. PMID 3012334.
- ↑ Enders G, Miller E, Cradock-Watson J, et al. (1994). "Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases". The Lancet. 343 (8912): 1548–51. doi:10.1016/S0140-6736(94)92943-2. PMID 7802767.
- ↑ Sørensen HT, Olsen JH, Jepsen P, et al. (2004). "The risk and prognosis of cancer after hospitalisation for herpes zoster: a population-based follow-up study". Br. J. Cancer. 91 (7): 1275–79. doi:10.1038/sj.bjc.6602120. PMC 2409892. PMID 15328522.
- ↑ Ragozzino MW, Melton LJ, Kurland LT, et al. (1982). "Risk of cancer after herpes zoster: a population-based study". The New England Journal of Medicine. 307 (7): 393–97. doi:10.1056/NEJM198208123070701. PMID 6979711.
- ↑ Ismail A, Rao DG, Sharrack B (2009). "Pure motor Herpes Zoster induced brachial plexopathy". Journal of Neurology. 256 (8): 1343–1345. doi:10.1007/s00415-009-5149-8. PMID 19434442.
- ↑ Roat MI (September 2014). "Herpes Zoster Ophthalmicus". Merck Manual. Archived from the original on 12 August 2016. Retrieved 14 August 2016.
- ↑ Apisarnthanarak A, Kitphati R, Tawatsupha P, et al. (2007). "Outbreak of varicella-zoster virus infection among Thai healthcare workers". Infect. Control Hosp. Epidemiol. 28 (4): 430–34. doi:10.1086/512639. PMID 17385149.
- ↑ 74.0 74.1 Araújo LQ, Macintyre CR, Vujacich C (2007). "Epidemiology and burden of herpes zoster and post-herpetic neuralgia in Australia, Asia and South America" (PDF). Herpes. 14 (Suppl 2): 40A–44A. PMID 17939895. Archived from the original (PDF) on 2019-12-05. Retrieved 2007-12-16.
- ↑ Brisson M, Edmunds WJ, Law B, et al. (2001). "Epidemiology of varicella zoster virus infection in Canada and the United Kingdom". Epidemiol. Infect. 127 (2): 305–14. doi:10.1017/S0950268801005921. PMC 2869750. PMID 11693508.
- ↑ Insinga RP, Itzler RF, Pellissier JM, et al. (2005). "The incidence of herpes zoster in a United States administrative database". J. Gen. Intern. Med. 20 (8): 748–53. doi:10.1111/j.1525-1497.2005.0150.x. PMC 1490195. PMID 16050886.
- ↑ Yawn BP, Saddier P, Wollan PC, et al. (2007). "A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction". Mayo Clin. Proc. 82 (11): 1341–49. doi:10.4065/82.11.1341. PMID 17976353.
- ↑ de Melker H, Berbers G, Hahné S, et al. (2006). "The epidemiology of varicella and herpes zoster in The Netherlands: implications for varicella zoster virus vaccination" (PDF). Vaccine. 24 (18): 3946–52. doi:10.1016/j.vaccine.2006.02.017. hdl:10029/5604. PMID 16564115. Archived (PDF) from the original on 2017-08-08. Retrieved 2019-09-03.
- ↑ Colebunders R, Mann JM, Francis H, et al. (1988). "Herpes zoster in African patients: a clinical predictor of human immunodeficiency virus infection". J. Infect. Dis. 157 (2): 314–18. doi:10.1093/infdis/157.2.314. PMID 3335810.
- ↑ Buchbinder SP, Katz MH, Hessol NA, et al. (1992). "Herpes zoster and human immunodeficiency virus infection". J. Infect. Dis. 166 (5): 1153–56. doi:10.1093/infdis/166.5.1153. PMID 1308664.
- ↑ Tsai SY, Chen HJ, Lio CF, et al. (2017-08-22). "Increased risk of herpes zoster in patients with psoriasis: A population-based retrospective cohort study". PLOS ONE. 12 (8): e0179447. Bibcode:2017PLoSO..1279447T. doi:10.1371/journal.pone.0179447. ISSN 1932-6203. PMC 5567491. PMID 28829784.
- ↑ Livengood JM (2000). "The role of stress in the development of herpes zoster and postherpetic neuralgia". Curr. Rev. Pain. 4 (1): 7–11. doi:10.1007/s11916-000-0003-9. PMID 10998709.
- ↑ 83.0 83.1 83.2 83.3 Gatti A, Pica F, Boccia MT, et al. (2010). "No evidence of family history as a risk factor for herpes zoster in patients with post-herpetic neuralgia". J. Med. Virol. 82 (6): 1007–11. doi:10.1002/jmv.21748. hdl:2108/15842. PMID 20419815.
- ↑ Schmader K, George LK, Burchett BM, et al. (1998). "Racial and psychosocial risk factors for herpes zoster in the elderly". J. Infect. Dis. 178 (Suppl 1): S67–S70. doi:10.1086/514254. PMID 9852978.
- ↑ Schmader K, George LK, Burchett BM, et al. (1998). "Race and stress in the incidence of herpes zoster in older adults". J. Am. Geriatr. Soc. 46 (8): 973–77. doi:10.1111/j.1532-5415.1998.tb02751.x. PMID 9706885.
- ↑ Hicks LD, Cook-Norris RH, Mendoza N, et al. (May 2008). "Family history as a risk factor for herpes zoster: a case-control study". Arch. Dermatol. 144 (5): 603–08. doi:10.1001/archderm.144.5.603. PMID 18490586.
- ↑ Marin M, Güris D, Chaves SS, et al. (June 22, 2007). "Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP)". MMWR Recomm. Rep. 56 (RR–4): 1–40. PMID 17585291. Archived from the original on September 4, 2011.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ Jumaan AO, Yu O, Jackson LA, et al. (2005). "Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002". J. Infect. Dis. 191 (12): 2002–07. doi:10.1086/430325. PMID 15897984.
- ↑ Whitley RJ (2005). "Changing dynamics of varicella-zoster virus infections in the 21st century: the impact of vaccination". J. Infect. Dis. 191 (12): 1999–2001. doi:10.1086/430328. PMID 15897983.
- ↑ Patel MS, Gebremariam A, Davis MM (December 2008). "Herpes zoster-related hospitalizations and expenditures before and after introduction of the varicella vaccine in the United States". Infect. Control Hosp. Epidemiol. 29 (12): 1157–63. doi:10.1086/591975. PMID 18999945.
- ↑ Yih WK, Brooks DR, Lett SM, et al. (2005). "The incidence of varicella and herpes zoster in Massachusetts as measured by the Behavioral Risk Factor Surveillance System (BRFSS) during a period of increasing varicella vaccine coverage, 1998–2003". BMC Public Health. 5: 68. doi:10.1186/1471-2458-5-68. PMC 1177968. PMID 15960856.
- ↑ Yawn BP, Saddier P, Wollan PC, et al. (2007). "A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction". Mayo Clin. Proc. 82 (11): 1341–49. doi:10.4065/82.11.1341. PMID 17976353.
- ↑ Volpi A (2007). "Severe complications of herpes zoster" (PDF). Herpes. 14 (Suppl 2): 35A–39A. PMID 17939894. Archived (PDF) from the original on 2019-01-27. Retrieved 2007-12-18.
- ↑ Coplan P, Black S, Rojas C, et al. (2001). "Incidence and hospitalization rates of varicella and herpes zoster before varicella vaccine introduction: a baseline assessment of the shifting epidemiology of varicella disease". Pediatr. Infect. Dis. J. 20 (7): 641–45. doi:10.1097/00006454-200107000-00002. PMID 11465834.
- ↑ Weaver BA (1 March 2007). "The burden of herpes zoster and postherpetic neuralgia in the United States". J. Am. Osteopath. Assoc. 107 (3 Suppl): S2–57. PMID 17488884. Archived from the original on 13 January 2008.
- ↑ Weller TH (2000). "Chapter 1. Historical perspective". In Arvin AM, Gershon AA (eds.). Varicella-Zoster Virus: Virology and Clinical Management. Cambridge University Press. ISBN 978-0-521-66024-2.
- ↑ Oaklander AL (October 1999). "The pathology of shingles: Head and Campbell's 1900 monograph". Arch. Neurol. 56 (10): 1292–94. doi:10.1001/archneur.56.10.1292. PMID 10520948.
- ↑ Weller TH (1953). "Serial propagation in vitro of agents producing inclusion bodies derived from varicella and herpes zoster". Proc. Soc. Exp. Biol. Med. 83 (2): 340–46. doi:10.3181/00379727-83-20354. PMID 13064265.
- ↑ Holt LE, McIntosh R (1936). Holt's Diseases of Infancy and Childhood. D Appleton Century Company. pp. 931–33.
- ↑ Weller TH (1997). "Varicella-herpes zoster virus". In Evans AS, Kaslow RA (eds.). Viral Infections of Humans: Epidemiology and Control. Plenum Press. pp. 865–92. ISBN 978-0-306-44855-3.
- ↑ Hope-Simpson RE (1965). "The nature of herpes zoster; a long-term study and a new hypothesis". Proceedings of the Royal Society of Medicine. 58 (1): 9–20. doi:10.1177/003591576505800106. PMC 1898279. PMID 14267505.
- ↑ Thomas SL, Wheeler JG, Hall AJ (2002). "Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study". The Lancet. 360 (9334): 678–82. doi:10.1016/S0140-6736(02)09837-9. PMID 12241874.
- ↑ Terada K, Hiraga Y, Kawano S, et al. (1995). "Incidence of herpes zoster in pediatricians and history of reexposure to varicella-zoster virus in patients with herpes zoster". Kansenshogaku Zasshi. 69 (8): 908–12. doi:10.11150/kansenshogakuzasshi1970.69.908. ISSN 0387-5911. PMID 7594784.
- ↑ ἕρπης. Liddell, Henry George; Scott, Robert; A Greek–English Lexicon at the Perseus Project.
- ↑ ἕρπειν in Liddell and Scott.
- ↑ Harper, Douglas. "herpes". Online Etymology Dictionary.
- ↑ "Herpes | Define Herpes at Dictionary.com". Archived from the original on 2011-02-25. Retrieved 2011-03-14.
- ↑ ζωστήρ in Liddell and Scott.
- ↑ Harper, Douglas. "zoster". Online Etymology Dictionary.
- ↑ cingulus, cingulum. Charlton T. Lewis and Charles Short. A Latin Dictionary on Perseus Project.
- ↑ Harper, Douglas. "shingles". Online Etymology Dictionary.
- ↑ Yawn BP, Gilden D (3 September 2013). "The global epidemiology of herpes zoster". Neurology. 81 (10): 928–30. doi:10.1212/wnl.0b013e3182a3516e. PMC 3885217. PMID 23999562.
- ↑ "Helvetesild (Herpes zoster)". helsenorge.no. Archived from the original on 22 December 2016. Retrieved 22 December 2016.
- ↑ 114.0 114.1 114.2 114.3 Becerra JC, Sieber R, Martinetti G, et al. (July 2013). "Infection of the central nervous system caused by varicella zoster virus reactivation: a retrospective case series study". International Journal of Infectious Diseases. 17 (7): e529–34. doi:10.1016/j.ijid.2013.01.031. PMID 23566589. Archived from the original on 2019-12-17. Retrieved 2016-03-02.
- ↑ Ihekwaba UK, Kudesia G, McKendrick MW (September 15, 2008). "Clinical features of viral meningitis in adults: significant differences in cerebrospinal fluid findings among herpes simplex virus, varicella zoster virus, and enterovirus infections" (PDF). Clinical Infectious Diseases. 47 (6): 783–9. doi:10.1086/591129. ISSN 1058-4838. PMID 18680414.
- ↑ 116.0 116.1 116.2 Pollak L, Dovrat S, Book M, et al. (August 2011). "Varicella zoster vs. herpes simplex meningoencephalitis in the PCR era. A single center study". Journal of the Neurological Sciences. 314 (1–2): 29–36. doi:10.1016/j.jns.2011.11.004. PMID 22138027.
- ↑ Kojima Y, Hashiguchi H, Hashimoto T, et al. (15 September 2008). "Recurrent Herpes Simplex Virus Type 2 Meningitis: A Case Report of Mollaret's Meningitis" (PDF). Japanese Journal of Infectious Diseases. 55 (3): 85–8. ISSN 1344-6304. PMID 12195049. Archived (PDF) from the original on 2013-01-22.
External links[edit | edit source]
| Classification | |
|---|---|
| External resources |
- NINDS Shingles Information Page Archived 2016-07-27 at the Wayback Machine, National Institute of Neurological Disorders and Stroke
Categories: [Chickenpox] [Oral mucosal pathology] [RTT] [RTTEM] [Varicella zoster virus-associated diseases] [Virus-related cutaneous conditions]
↧ Download as ZWI file | Last modified: 06/24/2024 10:24:46 | 229 views
☰ Source: https://mdwiki.org/wiki/Shingles | License: CC BY-SA 3.0
.jpg)
.jpg)
.jpg)
.jpg)
 KSF
KSF