Rhenium(Iii) Bromide
 From Handwiki
From Handwiki 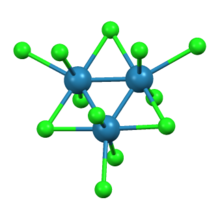
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
Re3Br9 |
| Molar mass | 425.92 g/mol |
| Appearance | Black lustrous solid[1] |
| Melting point | 500 °C (932 °F; 773 K)[2] (sublimes) |
| Solubility | Sparingly soluble in ether and acetone, reacts with methanol and ammonia[1] |
| Structure | |
Molecular shape
|
Trimeric |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
-164.4 kJ/mol[3] |
| Related compounds | |
Other anions
|
Rhenium(III) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhenium(III) bromide is a chemical compound with the formula Re3Br9. It is a black lustrous crystalline solid. This compound reacts with water to form rhenium(IV) oxide and is isostructural with rhenium(III) chloride.[1][4]
Preparation
This compound is prepared by the reaction of rhenium metal and bromine gas at 500 °C under nitrogen:[2]
- 6Re + 9Br2 → 2Re3Br9
If there is oxygen in the atmosphere, it will instead form rhenium(III) oxybromide.[2]
However, the most common method of producing this compound is by first reacting potassium hexabromorhenate(IV) with silver nitrate, which forms silver hexabromorhenite(IV), then this compound is heated to 600 °C to form rhenium(III) bromide.[1][3]
- K2ReBr6 + 2AgNO3 → Ag2ReBr6 + 2KNO3
- 6Ag2ReBr6 → 12AgBr + 3Br2 + 2Re3Br9
An alternative method is a thermal decomposition of rhenium(V) bromide.
References
- ↑ 1.0 1.1 1.2 1.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedrebr3 - ↑ 2.0 2.1 2.2 Harro Hagen; Adolf Sieverts (1933). "Rheniumtribromid" (in German). Zeitschrift für anorganische und allgemeine Chemie (Verlag GmbH & Co. KGaA, Weinheim) 215 (1): 111–112. doi:10.1002/zaac.19332150114.
- ↑ 3.0 3.1 J. P. King; J. W. Cobble (1960). "The Thermodynamic Properties of Technetium and Rhenium Compounds. VII. Heats of Formation of Rhenium Trichloride and Rhenium Tribromide. Free Energies and Entropies" (in English). Journal of the American Chemical Society 82 (9): 2111–2113. doi:10.1021/ja01494a005.
- ↑ V. V. Ugarov (1971). "Electron-diffraction investigation of the structure of the Re3Br9 molecule" (in English). Journal of Structural Chemistry 12 (2): 286–288. doi:10.1007/BF00739116.
 |
Categories: [Rhenium compounds] [Bromides]
↧ Download as ZWI file | Last modified: 02/20/2024 00:07:41 | 10 views
☰ Source: https://handwiki.org/wiki/Chemistry:Rhenium(III)_bromide | License: CC BY-SA 3.0

 KSF
KSF