Thyronine
 From Handwiki
From Handwiki 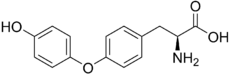
| |
| |
| Names | |
|---|---|
| IUPAC name
O-(4-Hydroxyphenyl)-L-tyrosine
| |
| Systematic IUPAC name
(2S)-2-Amino-3-[4-(4-hydroxyphenoxy)phenyl]propanoic acid | |
| Other names
4-(4-Hydroxyphenoxy)-L-phenylalanine
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C15H15NO4 |
| Molar mass | 273.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
- SizeSet
A group of metabolites derived from thyroxine and triiodothyronine via the peripheral enzymatic removal of iodines from the thyroxine nucleus. Thyronine is the thyroxine nucleus devoid of its four iodine atoms.[1]
References
- ↑ Pubchem Compound summary, L-thyronine
 |
Categories: [Alpha-Amino acids]
↧ Download as ZWI file | Last modified: 04/07/2025 05:13:48 | 2 views
☰ Source: https://handwiki.org/wiki/Chemistry:Thyronine | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF