Selenium Monochloride
 From Handwiki
From Handwiki 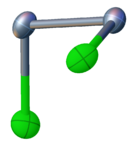
| |
| Names | |
|---|---|
| IUPAC name
Selenium monochloride
| |
| Other names
Dichlorodiselenide, Diselenium dichloride, Selenium chloride, 1,2-dichlorodiselane
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
Se2Cl2 |
| Molar mass | 228.83 g/mol |
| Appearance | Reddish-brown oily liquid |
| Density | 2.7741 g/cm3 |
| Melting point | −85 °C (−121 °F; 188 K) |
| Boiling point | 127 °C (261 °F; 400 K) at 0.997 atm |
Solubility in water
|
insoluble |
| Solubility in other solvents | Soluble in chloroform, carbon disulfide, and acetonitrile |
Magnetic susceptibility (χ)
|
−94.8·10−6 cm3/mol |
| Hazards | |
| GHS pictograms |    
|
| GHS Signal word | Danger |
GHS hazard statements
|
H301, H311, H314, H331, H373, H410 |
GHS precautionary statements
|
P260, P261, P264, P270, P271, P273, P280, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P311, P312, P314, P321, P322, P330, P361, P363, P391, P403+233, P405 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
Selenium monochloride is an inorganic compound with the formula Se2Cl2. Although it is called selenium monochloride, a more descriptive name might be diselenium dichloride. It is a reddish-brown, oily liquid that hydrolyses slowly. It exists in chemical equilibrium with SeCl2, SeCl4, chlorine, and elemental selenium.[1] Selenium monochloride is mainly used as a reagent for the synthesis of Se-containing compounds.
Structure and properties
Selenium monochloride has the connectivity Cl-Se-Se-Cl. With a nonplanar structure, it has C2 molecular symmetry, similar to hydrogen peroxide and sulfur monochloride. The Se-Se bond length is 2.23 Å, and the Se-Cl bond lengths are 2.20 Å. The dihedral angle is 87°.[2]
Preparation
Early routes to selenium monochloride involved chlorination of elemental selenium.[3] An improved method involves the reaction of a mixture of selenium, selenium dioxide, and hydrochloric acid:[4]
- 3 Se + SeO2 + 4 HCl → 2 Se2Cl2 + 2 H2O
A dense layer of selenium monochloride settles from the reaction mixture, which can be purified by dissolving it in fuming sulfuric acid and reprecipitating it with hydrochloric acid. A second method for the synthesis involves the reaction of selenium with oleum and hydrochloric acid:[4]
- 2 Se + 2 SO3 + 3 HCl → Se2Cl2 + H2SO3 + SO2(OH)Cl
The crude selenium monochloride is removed via separatory funnel. Selenium monochloride cannot be distilled without decomposition, even at reduced pressure.[4]
In acetonitrile solutions, it exists in equilibrium with SeCl2 and SeCl4.[5] Selenium dichloride degrades to the monochloride after a few minutes at room temperature:[6]
- 3 SeCl2 → Se2Cl2 + SeCl4
Reactions
Selenium monochloride is an electrophilic selenizing agent, and thus it reacts with simple alkenes to give bis(β-chloroalkyl)selenide and bis(chloroalkyl)selenium dichloride. It converts hydrazones of hindered ketones into the corresponding selenoketones, the structural analogs of ketones whereby the oxygen atom is replaced with a selenium atom.[7] Finally, the compound has been used to introduce bridging selenium ligands between the metal atoms of some iron and chromium carbonyl complexes.[7]
References
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Kniep, Rüdiger; Körte, Lutz; Mootz, Dietrich (1 January 1983). "Kristallstrukturen von Verbindungen A2X2 (A = S, Se; X = Cl, Br)". Zeitschrift für Naturforschung B 38 (1): 1–6. doi:10.1515/znb-1983-0102.
- ↑ Lenher, Victor; Kao, C. H. (1925). "The Preparation of Selenium Monochloride and Monobromide". Journal of the American Chemical Society 47 (3): 772–774. doi:10.1021/ja01680a025.
- ↑ 4.0 4.1 4.2 Fehér, F. "Diselenium Dichloride". In Handbook of Preparative Inorganic Chemistry; Brauer, G., Ed.; Academic Press: New York, 1963; Vol. 1; p 422-433.
- ↑ Lamoureux, Marc; Milne, John (1990). "Selenium chloride and bromide equilibria in aprotic solvents; a 77Se NMR study". Polyhedron 9 (4): 589–595. doi:10.1016/S0277-5387(00)86238-5.
- ↑ Maaninen, Arto; Chivers, Tristram; Parvez, Masood; Pietikäinen, Jarkko; Laitinen, Risto S. (1999). "Syntheses of THF Solutions of SeX2(X = Cl, Br) and a New Route to Selenium Sulfides SenS8−n(n = 1−5): X-ray Crystal Structures of SeCl2(tht)2 and SeCl2·tmtu". Inorganic Chemistry 38 (18): 4093–4097. doi:10.1021/ic981430h.
- ↑ 7.0 7.1 Back, Thomas G.; Moussa, Ziad (2003). "Encyclopedia of Reagents for Organic Synthesis". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rn00201. ISBN 0471936235.
 |
Categories: [Selenium compounds] [Chlorides] [Nonmetal halides]
↧ Download as ZWI file | Last modified: 09/18/2023 12:46:26 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Selenium_monochloride | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF