2-(2-(4-Methyl-3-Cyclohexen-1-Yl)Propyl)Cyclopentanone
 From Handwiki
From Handwiki 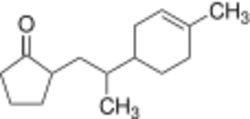 Structure without shown stereo chemistry
| |
| Names | |
|---|---|
| Preferred IUPAC name
2-[2-(4-Methylcyclohex-3-en-1-yl)propyl]cyclopentan-1-one | |
| Other names
methylcyclohexenylpropyl-cyclopentanone (INCI); Nectaryl
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEMBL |
|
| ChemSpider |
|
| EC Number |
|
PubChem CID
|
|
| UNII |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C15H24O |
| Molar mass | 220.35 g·mol−1 |
| Appearance | viscous liquid with fruity odor[1] |
| Density | 0.96 g·cm−3 (22 °C)[1] |
| Melting point | −41.8 °C (−43.2 °F; 231.3 K)[1] |
| Boiling point | 288 °C (550 °F; 561 K)[1] |
Solubility in water
|
practically insoluble in water (4.6 mg·l−1 at 20 °C)[1] |
| Hazards | |
| Safety data sheet | [1] |
| GHS pictograms | 
|
| GHS Signal word | Warning |
GHS hazard statements
|
H410 |
GHS precautionary statements
|
P273, P391, P501 |
| Lethal dose or concentration (LD, LC): | |
LC50 (median concentration)
|
5.47 mg·L−1 (zebrafish) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
2-[2-(4-Methyl-3-cyclohexen-1-yl)propyl]cyclopentanone (trade name by Givaudan: Nectaryl) is an organic compound belonging to the group of ketones and cycloalkanes. The compound is used as a fragrance.
Synthesis
The synthesis of the compound is carried out by a radical addition of cyclopentanone to (+)-limonene under oxygen in acetic acid. As a catalyst, manganese(II) acetate and cobalt(II) acetate are used.[2]
Properties
The flash point of the compound is 162.5 °C, and the autoignition temperature is 294 °C.[1] The specific rotation is reported to be [α]D20=+228–235° (1 M; chloroform)[2]
In general, the compound features a fruity apricot-like odor. Of the four stereo isomers, (2R,2′S,1′′R)-Nectaryl and (2R,2′R,1′′R)-Nectaryl contribute especially to the compound's odor, the odor detection threshold lies at 0.094 ng·l−1 and 0.112 ng·l−1, respectively. In contrast to that, the other stereo isomers show an unspecific fruity odor, the odor detection threshold are 11.2 ng·l−1 and 14.9 ng·l−1 which is much higher.[2][3]
The tenacity on blotter (the time during which the compound is smellable with unchanged characteristics[4]) is reported to be three weeks.[5]
Uses
The substance is used as a fragrance in exemplary air conditioning products, perfumes and polishes.[6]
Literature
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Record of 2-[2-(4-Methyl-3-cyclohexen-1-yl)propylcyclopentanon] in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 3. April 2019.
- ↑ 2.0 2.1 2.2 Elisabetta Brenna, Claudio Fuganti, Francesco G. Gatti, Luciana Malpezzi, Stefano Serra (2008). "Synthesis and olfactory evaluation of all stereoisomers of the fragrance Nectaryl". Tetrahedron: Asymmetry 19 (7): 800–807. doi:10.1016/j.tetasy.2008.03.011.
- ↑ John C. Leffingwell. "Chirality & Odour Perception". leffingwell.com. http://www.leffingwell.com/chirality/nectaryl.htm.
- ↑ Wolfgang Legrum (2015). "3" (in de). Riechstoffe, zwischen Gestank und Duft (2 ed.). Springer. pp. 51. ISBN 978-3-658-07310-7.
- ↑ Wolfgang Legrum (2015). "3" (in de). Riechstoffe, zwischen Gestank und Duft (2 ed.). Springer. pp. 52. ISBN 978-3-658-07310-7. https://books.google.com/books?id=g-ndBgAAQBAJ&pg=PA52. Retrieved 2019-04-03.
- ↑ ECHA, ed. "2-(2-(4-methyl-3-cyclohexen-1-yl)propyl)cyclopentanone". https://echa.europa.eu/de/substance-information/-/substanceinfo/100.100.590.
 |
Categories: [Cyclohexenes] [Cyclic ketones] [Perfume ingredients]
↧ Download as ZWI file | Last modified: 02/12/2024 15:07:48 | 12 views
☰ Source: https://handwiki.org/wiki/Chemistry:2-(2-(4-Methyl-3-cyclohexen-1-yl)propyl)cyclopentanone | License: CC BY-SA 3.0

 KSF
KSF