Malvin
|
| Names
|
| IUPAC name
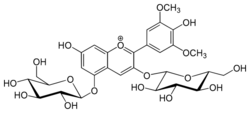
3,5-Bis(β-D-glucopyranosyloxy)-4′,7-dihydroxy-3′,5′-dimethoxyflavylium
|
Systematic IUPAC name
7-Hydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3,5-bis{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ4-benzopyran-1-ylium |
| Other names
Malvidin 3,5-diglucoside
|
| Identifiers
|
CAS Number
|
- 16727-30-3
 Y Y
|
3D model (JSmol)
|
- (cation): Interactive image
- (chloride): Interactive image
|
| ChEBI
|
- CHEBI:75030
 N N
|
| ChemSpider
|
- 390365 (cation)
 N N - 16498815 (chloride)
 N N
|
| KEGG
|
- C08718
 N N
|
|
|
|
| UNII
|
- I9I120531L
 Y Y
|
InChI
(cation): InChI=1S/C29H34O17/c1-40-15-3-10(4-16(41-2)20(15)33)27-17(44-29-26(39)24(37)22(35)19(9-31)46-29)7-12-13(42-27)5-11(32)6-14(12)43-28-25(38)23(36)21(34)18(8-30)45-28/h3-7,18-19,21-26,28-31,34-39H,8-9H2,1-2H3,(H-,32,33)/p+1/t18-,19-,21-,22-,23+,24+,25-,26-,28-,29-/m1/s1 Key: CILLXFBAACIQNS-BTXJZROQSA-O (chloride): InChI=1S/C29H34O17.ClH/c1-40-15-3-10(4-16(41-2)20(15)33)27-17(44-29-26(39)24(37)22(35)19(9-31)46-29)7-12-13(42-27)5-11(32)6-14(12)43-28-25(38)23(36)21(34)18(8-30)45-28;/h3-7,18-19,21-26,28-31,34-39H,8-9H2,1-2H3,(H-,32,33);1H/t18-,19-,21-,22-,23+,24+,25-,26-,28-,29-;/m1./s1 Key: RHKJIVJBQJXLBY-FTIBDFQESA-N
|
SMILES
(cation): COC1=CC(=CC(=C1O)OC)C2=C(C=C3C(=CC(=CC3=[O+]2)O)O[C@H]4[C@@H]([C@H]([C@@H]([C@H](O4)CO)O)O)O)O[C@H]5[C@@H]([C@H]([C@@H]([C@H](O5)CO)O)O)O (chloride): [Cl-].O[C@@H]5[C@@H](O)[C@H](O)[C@@H](CO)O[C@H]5Oc2cc(O)cc3[o+]c(c(O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)cc23)c4cc(OC)c(O)c(OC)c4
|
| Properties
|
Chemical formula
|
- C29H35O17+ (cation)
- C29H35O17Cl (chloride)
|
| Molar mass
|
- 655.578 mg/L (cation)
- 691.031 mg/L (chloride)
|
| Appearance
|
Reddish blue, odorless powder[1]
|
Solubility in water
|
Nearly insoluble[1]
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?)
|
| Infobox references
|
|
|
|
Tracking categories (test):
Malvin is a naturally occurring chemical of the anthocyanin family.
Malvin reacts in the presence of H2O2 to form malvone.[2] The ortho-benzoyloxyphenylacetic acid esters reaction product is dependant of the pH: it is obtained under acidic conditions whereas under neutral conditions, the reaction product is the 3-O-acyl-glucosyl-5-O-glucosyl-7-hydroxy coumarin.[3]
Natural occurrences
It is a diglucoside of malvidin mainly found as a pigment in herbs like Malva (Malva sylvestris), Primula and Rhododendron.[4] M. sylvestris also contains malonylmalvin (malvidin 3-(6″-malonylglucoside)-5-glucoside).[5]
The characteristic floral jade coloration of Strongylodon macrobotrys has been shown to be an example of copigmentation, a result of the presence of malvin and saponarin (a flavone glucoside) in the ratio 1:9.
Presence in food
Malvin can be found in a variety of common foods, including peaches (Clingstone variety[6]).
References
- ↑ 1.0 1.1 MSDS from CarlRoth (German)
- ↑ Oxidation of the anthocyanidin-3,5-diglucosides with H2O2: The structure of malvone. G. Hrazdina, Phytochemistry, July 1970, Volume 9, Issue 7, Pages 1647–1652, doi:10.1016/S0031-9422(00)85290-5
- ↑ Oxidation products of acylated anthocyanins under acidic and neutral conditions. Géza Hrazdina and Angeline J. Franzese, Phytochemistry, January 1974, Volume 13, Issue 1, Pages 231–234, doi:10.1016/S0031-9422(00)91300-1
- ↑ J. A. Joule, K. Mills: Heterocyclic Chemistry., S. 173, Blackwell Publishing, 2000, ISBN:978-0-632-05453-4
- ↑ Malonated anthocyanins in malvaceae: Malonylmalvin from Malva sylvestris. Kosaku Takeda, Shigeki Enoki, Jeffrey B. Harborne and John Eagles, Phytochemistry, 1989, Volume 28, Issue 2, Pages 499–500, doi:10.1016/0031-9422(89)80040-8
- ↑ Chang, S; Tan, C; Frankel, EN; Barrett, DM (2000). "Low-density lipoprotein antioxidant activity of phenolic compounds and polyphenol oxidase activity in selected clingstone peach cultivars". Journal of Agricultural and Food Chemistry 48 (2): 147–51. doi:10.1021/jf9904564. PMID 10691607.
Anthocyanidins and their anthocyanin glucosides |
|---|
| 3-Hydroxyanthocyanidins |
- 5-Desoxy-peonidin
- Aurantinidin
- Cyanidin
- 6-Hydroxycyanidin
- Delphinidin
- Fisetinidin
- Guibourtinidin
- Pelargonidin
- Robinetinidin
|
|---|
| 3-Deoxyanthocyanidins |
- Apigeninidin
- Columnidin
- Diosmetinidin
- Gesneridin
- Luteolinidin
- Tricetinidin
|
|---|
| O-Methylated anthocyanidins |
- 5-Desoxy-malvidin
- Capensinidin
- Europinidin
- Hirsutidin
- Kaempferidinidin
- Malvidin
- Peonidin
- Petunidin
- Pulchellidin
- Rosinidin
|
|---|
Anthocyanins
(anthocyaninidin glycosides) | Glucosides:
- Callistephin (Pelargonidin 3-O-glucoside)
- Chrysanthemin (Cyanidin 3-O-glucoside)
- Myrtillin (Delphinidin 3-O-glucoside)
- Oenin (Malvidin 3-O-glucoside)
- Peonidin 3-O-glucoside
- Petunidin 3-O-glucoside
- Pulchellidin 3-glucoside
Diglucosides:
- Cyanin (Cyanidin 3,5-O-diglucoside)
- Delphin (Delphinidin 3,5-O-diglucoside)
- Malvin (Malvidin 3,5-diglucoside)
- Pelargonin (Pelargonidin 3,5-O-diglucoside)
- Peonin (Peonidin 3,5-O-diglucoside)
- Petunin (Petunidin 3,5-O-diglucoside)
Others glycosides:
- Antirrhinin (Cyanidin 3-O-rutinoside)
- Ideain (Cyanidin 3-O-galactoside)
- Delphinidin 3-O-rhamnoside
- Petunidin 3-O-arabinoside
- Petunidin 3-O-galactoside
- Petunidin 3-O-rhamnoside
- Petunidin 3-O-rutinoside
- Primulin (Malvidin 3-O-galactoside)
- Pulchellidin 3-rhamnoside
- Tulipanin (Delphinidin 3-O-rutinoside)
|
|---|
| Acylated anthocyanins | | Acetylated anthocyanins |
Cyanidin 3-O-(6-acetyl)glucoside
Delphinidin 3-O-(6-acetyl)glucoside
Malvidin 3-O-(6-acetyl)glucoside
Petunidin 3-O-(6-acetyl)galactoside
Petunidin 3-O-(6-acetyl)glucoside
Peonidin 3-O-(6-acetyl)glucoside
|
|---|
Coumaroylated anthocyanins
(cis- and trans-) |
- Cyanidin 3-O-(6-p-coumaroyl)glucoside
- Delphinidin 3-O-(6-p-coumaroyl)glucoside
- Malvidin 3-O-(6-p-coumaroyl)glucoside
- Petunidin 3-O-(6-p-coumaroyl)glucoside
- Peonidin 3-O-(6-p-coumaroyl)glucoside
|
|---|
| Caffeoylated anthocyanins |
- Malvidin 3-O-(6-p-caffeoyl)glucoside
- Peonidin 3-O-(6-p-caffeoyl)glucoside
|
|---|
| Malonylated anthocyanins |
- Malonylmalvin (malvidin 3-(6″-malonylglucoside)-5-glucoside)
|
|---|
| Acylated anthocyanin diglycosides |
- Cyanidin 3-O-(di-p-coumarylglucoside)-5-glucoside
- Gentiodelphin (delphinidin 3-''O''-glucosyl-5-''O''-(6-''O''-caffeoyl-glucosyl)-3′-''O''-(6-''O''-caffeoyl-glucoside))
- Nasunin (Delphinidin 3-(p-coumaroylrutinoside)-5-glucoside)
- Petanin (petunidin 3-[6-O-(4-O-(E)-p-coumaroyl-O-α-l-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-β-D-glucopyranoside)
- Violdelphin (Delphinidin 3-rutinoside-7-O-(6-O-(4-(6-O-(4-hydroxybenzoyl)-β-D-glucosyl)oxybenzoyl)-β-D-glucoside)
|
|---|
|
|---|
| Flavanol-anthocyanin adducts |
- Malvidin glucoside-ethyl-catechin
- Catechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Epicatechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Afzelechin(4α→8)pelargonidin 3-O-β-glucopyranoside
- Epiafzelechin(4α→8)pelargonidin 3-O-β-glucopyranoside
|
|---|
| Miscellaneous |
- Metalloanthocyanins (commelinin)
- Cyanosalvianin
- Protocyanin
- Protodelphin)
- Pyranoanthocyanins
- Copigmentation
- Anthocyanone A (degradation product of oenin)
- Malvone (oxidation product of malvin)
|
|---|
 | Original source: https://en.wikipedia.org/wiki/Malvin. Read more |
 From Handwiki
From Handwiki 


 KSF
KSF