Short description: Chemical compound
1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine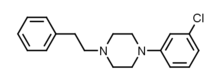 |
| Identifiers |
|---|
IUPAC name
1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine
|
| CAS Number | - 136534-45-7
 Y Y
|
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| UNII | |
|---|
| Chemical and physical data |
|---|
| Formula | C18H21ClN2 |
|---|
| Molar mass | 300.83 g·mol−1 |
|---|
| 3D model (JSmol) | |
|---|
SMILES
ClC1=CC=CC(=C1)N2CCN(CC2)CCC3=CC=CC=C3
|
InChI
InChI=1S/C18H21ClN2/c19-17-7-4-8-18(15-17)21-13-11-20(12-14-21)10-9-16-5-2-1-3-6-16/h1-8,15H,9-14H2 Key:NKMGWZZAFWDLFG-UHFFFAOYSA-N
|
1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine (3C-PEP) is a designer drug of the piperazine class of chemical substances. 3C-PEP is related to meta-cholorophenylpiperazine (mCPP) and phenethylamine that can be thought of as mCPP having a phenylethyl group attached to the nitrogen atom at its 4-position. It was first described in 1994 in a patent disclosing a series of piperazine compounds as sigma receptor ligands.[1] Later, it was discovered to be a highly potent dopamine reuptake inhibitor.[2]
Pharmacology
3C-PEP is one of the most potent dopamine transporter (DAT) ligand reported to date. It is highly selective for the dopamine transporter (dissociation constant Ki = 0.04 nM) with relatively low affinity for the closely related norepinephrine transporter (NET, Ki = 1107 nM ) and the serotonin transporter (SERT, Ki = 802 nM). In addition, the compound has lower (or no) affinity for D2-like receptor (Ki = 327 nM), serotonin 5-HT2 receptor (Ki = 53 nM), opioid receptor (Ki > 10000 nM), and the PCP/NMDA receptor (Ki > 10000 nM).[2]
With a DAT dissociation constant Ki of 0.04 nM, 3C-PEP is one of the most potent dopamine transporter ligand described to date in the literature. In comparison, cocaine which is a prototypical DAT ligand and reuptake inhibitor has a dissociation constant Ki of 435 nm thus making 3C-PEP about 10,000 times more potent than cocaine as a dopamine transporter inhibitor in vitro.[2]
Legal status
United States
3C-PEP is not scheduled at the federal level in the United States ,[3]
Canada
3C-PEP is not scheduled under the Controlled Drugs and Substances Act.
See also
- Substituted piperazine
- Etoperidone, nefazodone, and trazodone (structurally related drugs which may also produce mCPP as a metabolite)
- CM156
- Diphenpipenol
- MT-45
References
- ↑ Glennon, Richard A. "Sigma receptor ligands and the use thereof". http://www.google.com/patents/US6057371.
- ↑ 2.0 2.1 2.2 "Chlorophenylpiperazine analogues as high affinity dopamine transporter ligands". Bioorg Med Chem Lett 23 (24): 6020–6922. 2013. doi:10.1016/j.bmcl.2013.09.038. PMID 24211020.
- ↑ "21 CFR — SCHEDULES OF CONTROLLED SUBSTANCES §1308.11 Schedule I.". http://www.deadiversion.usdoj.gov/21cfr/cfr/1308/1308_11.htm.
Stimulants |
|---|
| Adamantanes |
- Adapromine
- Amantadine
- Bromantane
- Memantine
- Rimantadine
|
|---|
| Adenosine antagonists |
- 8-Chlorotheophylline
- 8-Cyclopentyltheophylline
- 8-Phenyltheophylline
- Aminophylline
- Caffeine
- CGS-15943
- Dimethazan
- Paraxanthine
- SCH-58261
- Theobromine
- Theophylline
|
|---|
| Alkylamines |
- Cyclopentamine
- Cypenamine
- Cyprodenate
- Heptaminol
- Isometheptene
- Levopropylhexedrine
- Methylhexaneamine
- Octodrine
- Propylhexedrine
- Tuaminoheptane
|
|---|
| Ampakines |
- CX-516
- CX-546
- CX-614
- CX-691
- CX-717
- IDRA-21
- LY-404,187
- LY-503,430
- Nooglutyl
- Org 26576
- PEPA
- S-18986
- Sunifiram
- Unifiram
|
|---|
| Arylcyclohexylamines |
- Benocyclidine
- Dieticyclidine
- Esketamine
- Eticyclidine
- Gacyclidine
- Ketamine
- Phencyclamine
- Phencyclidine
- Rolicyclidine
- Tenocyclidine
- Tiletamine
|
|---|
| Benzazepines |
- 6-Br-APB
- SKF-77434
- SKF-81297
- SKF-82958
|
|---|
| Cholinergics |
- A-84,543
- A-366,833
- ABT-202
- ABT-418
- AR-R17779
- Altinicline
- Anabasine
- Arecoline
- Bradanicline
- Cotinine
- Cytisine
- Dianicline
- Epibatidine
- Epiboxidine
- GTS-21
- Ispronicline
- Nicotine
- PHA-543,613
- PNU-120,596
- PNU-282,987
- Pozanicline
- Rivanicline
- Sazetidine A
- SIB-1553A
- SSR-180,711
- TC-1698
- TC-1827
- TC-2216
- Tebanicline
- UB-165
- Varenicline
- WAY-317,538
|
|---|
| Convulsants |
- Anatoxin-a
- Bicuculline
- DMCM
- Flurothyl
- Gabazine
- Haldol
- Pentetrazol
- Picrotoxin
- Strychnine
- Thujone
|
|---|
| Eugeroics |
- Adrafinil
- Armodafinil
- CRL-40,940
- CRL-40,941
- Fluorenol
- Modafinil
|
|---|
| Oxazolines |
- 4-Methylaminorex
- Aminorex
- Clominorex
- Cyclazodone
- Fenozolone
- Fluminorex
- Pemoline
- Thozalinone
|
|---|
| Phenethylamines |
- 1-(4-Methylphenyl)-2-aminobutane
- 1-Methylamino-1-(3,4-methylenedioxyphenyl)propane
- 2-Fuoroamphetamine
- 2-Fuoromethamphetamine
- 2-OH-PEA
- 2-Phenyl-3-aminobutane
- 2,3-MDA
- 3-Fuoroamphetamine
- 3-Fluoroethamphetamine
- 3-Fluoromethcathinone
- 3-Methoxyamphetamine
- 3-Methylamphetamine
- 3,4-DMMC
- 4-BMC
- 4-CMC
- 4-Fluoroamphetamine
- 4-Fluoromethamphetamine
- 4-MA
- 4-Methylbuphedrone
- 4-Methylcathinone
- 4-MEAP
- 4-MMA
- 4-Methylpentedrone
- 4-MTA
- 6-FNE
- AL-1095
- Alfetamine
- a-Ethylphenethylamine
- Amfecloral
- Amfepentorex
- Amfepramone
- Amidephrine
- 2-Amino-1,2-dihydronaphthalene
- 2-Aminoindane
- 5-(2-Aminopropyl)indole
- 2-Aminotetralin
- Acridorex
- Amphetamine (Dextroamphetamine, Levoamphetamine)
- Amphetaminil
- Arbutamine
- β-Methylphenethylamine
- β-Phenylmethamphetamine
- Benfluorex
- Benzedrone
- Benzphetamine
- BDB
- BOH
- 3-Benzhydrylmorpholine
- BPAP
- Buphedrone
- Bupropion
- Butylone
- Camfetamine
- Cathine
- Cathinone
- Chlorphentermine
- Cilobamine
- Cinnamedrine
- Clenbuterol
- Clobenzorex
- Cloforex
- Clortermine
- Cypenamine
- D-Deprenyl
- Denopamine
- Dimethoxyamphetamine
- Dimethylamphetamine
- Dimethylcathinone
- Dobutamine
- DOPA (Dextrodopa, Levodopa)
- Dopamine
- Dopexamine
- Droxidopa
- EBDB
- Ephedrine
- Epinephrine
- Epinine
- Etafedrine
- Ethcathinone
- Ethylnorepinephrine
- Ethylone
- Etilamfetamine
- Etilefrine
- Famprofazone
- Fencamfamin
- Fencamine
- Fenethylline
- Fenfluramine (Dexfenfluramine, Levofenfluramine)
- Fenproporex
- Feprosidnine
- Flephedrone
- Fludorex
- Formetorex
- Furfenorex
- Gepefrine
- Hexapradol
- Hexedrone
- HMMA
- Hordenine
- 4-Hydroxyamphetamine
- 5-Iodo-2-aminoindane
- Ibopamine
- Indanylamphetamine
- Iofetamine
- Isoetarine
- Isoethcathinone
- Isoprenaline
- L-Deprenyl (Selegiline)
- Lefetamine
- Lisdexamfetamine
- Lophophine
- MBDB
- MDA (tenamfetamine)
- MDBU
- MDEA
- MDMA (midomafetamine)
- MDMPEA
- MDOH
- MDPR
- MDPEA
- Mefenorex
- Mephedrone
- Mephentermine
- Metanephrine
- Metaraminol
- Mesocarb
- Methamphetamine (Dextromethamphetamine, Levomethamphetamine)
- Methoxamine
- Methoxyphenamine
- MMA
- Methcathinone
- Methedrone
- Methoxyphenamine
- Methylenedioxycathinone
- Methylone
- Mexedrone
- MMDA
- MMDMA
- MMMA
- Morforex
- N,alpha-Diethylphenylethylamine
- N-Ethylbuphedrone
- N-Ethylhexedrone
- N,N-Dimethylphenethylamine
- Naphthylamphetamine
- Nisoxetine
- Norepinephrine
- Norfenefrine
- Norfenfluramine
- Normetanephrine
- L-Norpseudoephedrine
- Octopamine (drug)
- Orciprenaline
- Ortetamine
- Oxifentorex
- Oxilofrine
- PBA
- PCA
- PCMA
- PHA
- Pentorex
- Pentedrone
- Pentylone
- Phenatine
- Phenpromethamine
- Phentermine
- Phenylalanine
- Phenylephrine
- Phenylpropanolamine
- Pholedrine
- PIA
- PMA
- PMEA
- PMMA
- PPAP
- Phthalimidopropiophenone
- Prenylamine
- Propylamphetamine
- Pseudoephedrine
- Ropinirole
- Salbutamol (Levosalbutamol)
- Sibutramine
- Solriamfetol
- Synephrine
- Theodrenaline
- Tiflorex
- Tranylcypromine
- Tyramine
- Tyrosine
- Xylopropamine
- Zylofuramine
|
|---|
| Phenylmorpholines |
- 3-Fluorophenmetrazine
- Fenbutrazate
- Fenmetramide
- G-130
- Manifaxine
- Morazone
- Morforex
- Oxaflozane
- PD-128,907
- Phendimetrazine
- Phenmetrazine
- 2-Phenyl-3,6-dimethylmorpholine
- Pseudophenmetrazine
- Radafaxine
|
|---|
| Piperazines |
- 2C-B-BZP
- 3C-PEP
- BZP
- CM156
- DBL-583
- GBR-12783
- GBR-12935
- GBR-13069
- GBR-13098
- GBR-13119
- MeOPP
- MBZP
- oMPP
- Vanoxerine
|
|---|
| Piperidines |
- 1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine
- 2-Benzylpiperidine
- 2-Methyl-3-phenylpiperidine
- 3,4-Dichloromethylphenidate
- 4-Benzylpiperidine
- 4-Fluoromethylphenidate
- 4-Methylmethylphenidate
- Desoxypipradrol
- Difemetorex
- Diphenylpyraline
- Ethylnaphthidate
- Ethylphenidate
- Methylnaphthidate
- Isopropylphenidate
- JZ-IV-10
- Methylphenidate (Dexmethylphenidate)
- Nocaine
- Phacetoperane
- Pipradrol
- Propylphenidate
- SCH-5472
|
|---|
| Pyrrolidines |
- 2-Diphenylmethylpyrrolidine
- 5-DBFPV
- α-PPP
- α-PBP
- α-PHP
- α-PHPP
- α-PVP
- α-PVT
- Diphenylprolinol
- DMPVP
- FPOP
- FPVP
- MDPPP
- MDPBP
- MPBP
- MPHP
- MPPP
- MOPVP
- MOPPP
- Indapyrophenidone
- MDPV
- Naphyrone
- PEP
- Picilorex
- Prolintane
- Pyrovalerone
|
|---|
| Racetams |
- Oxiracetam
- Phenylpiracetam
- Phenylpiracetam hydrazide
|
|---|
| Tropanes |
- 4-fluorotropacocaine
- 4'-Fluorococaine
- Altropane (IACFT)
- Brasofensine
- CFT (WIN 35,428)
- β-CIT (RTI-55)
- Cocaethylene
- Cocaine
- Dichloropane (RTI-111)
- Difluoropine
- FE-β-CPPIT
- FP-β-CPPIT
- Ioflupane (123I)
- Norcocaine
- PIT
- PTT
- RTI-31
- RTI-32
- RTI-51
- RTI-112
- RTI-113
- RTI-120
- RTI-121 (IPCIT)
- RTI-126
- RTI-150
- RTI-177
- RTI-229
- RTI-336
- RTI-354
- RTI-371
- RTI-386
- Salicylmethylecgonine
- Tesofensine
- Troparil (β-CPT, WIN 35,065-2)
- Tropoxane
- WF-23
- WF-33
|
|---|
| Tryptamines |
- 4-HO-αMT
- 4-Methyl-αET
- 4-Methyl-αMT
- 5-Chloro-αMT
- 5-Fluoro-αMT
- 5-MeO-αET
- 5-MeO-αMT
- 5-MeO-DIPT
- 6-Fluoro-αMT
- 7-Methyl-αET
- αET
- αMT
|
|---|
| Others |
- 2-MDP
- 3,3-Diphenylcyclobutanamine
- Amfonelic acid
- Amineptine
- Amiphenazole
- Atipamezole
- Atomoxetine
- Bemegride
- Benzydamine
- BTQ
- BTS 74,398
- Centanafadine
- Ciclazindol
- Clofenciclan
- Cropropamide
- Crotetamide
- D-161
- Desipramine
- Diclofensine
- Dimethocaine
- Efaroxan
- Etamivan
- Fenisorex
- Fenpentadiol
- Gamfexine
- Gilutensin
- GSK1360707F
- GYKI-52895
- Hexacyclonate
- Idazoxan
- Indanorex
- Indatraline
- JNJ-7925476
- Lazabemide
- Leptacline
- Lomevactone
- LR-5182
- Mazindol
- Meclofenoxate
- Medifoxamine
- Mefexamide
- Methamnetamine
- Methastyridone
- Methiopropamine
- Naphthylaminopropane
- Nefopam
- Nikethamide
- Nomifensine
- O-2172
- Oxaprotiline
- PNU-99,194
- PRC200-SS
- Rasagiline
- Rauwolscine
- Rubidium chloride
- Setazindol
- Tametraline
- Tandamine
- Thiopropamine
- Thiothinone
- Trazium
- UH-232
- Yohimbine
|
|---|
ATC code: N06B |
Dopamine receptor modulators |
|---|
| D1-like | | Agonists |
- Benzazepines: 6-Br-APB
- Fenoldopam
- SKF-38,393
- SKF-77,434
- SKF-81,297
- SKF-82,958
- SKF-83,959
- Trepipam
- Zelandopam
- Ergolines: Cabergoline
- CY-208,243
- Dihydroergocryptine
- LEK-8829
- Lisuride
- Pergolide
- Terguride
- Dihydrexidine derivatives: A-77636
- A-86929
- Adrogolide (ABT-431, DAS-431)
- Dihydrexidine
- Dinapsoline
- Dinoxyline
- Doxanthrine
- Phenethylamines: BCO-001
- Deoxyepinephrine (N-methyldopamine, epinine)
- Dopexamine
- Etilevodopa
- Ibopamine
- L-DOPA (levodopa)
- Melevodopa
- L-Phenylalanine
- L-Tyrosine
- XP21279
- Others: A-68930
- Apomorphine
- Isocorypalmine
- Nuciferine
- PF-6649751
- PF 6669571
- Propylnorapomorphine
- Rotigotine
- SKF-89,145
- SKF-89,626
- Stepholidine
- Tetrahydropalmatine
|
|---|
| Antagonists |
- Typical antipsychotics: Butaclamol
- Chlorpromazine
- Chlorprothixene
- Flupentixol (flupenthixol) (+melitracen)
- Fluphenazine
- Loxapine
- Perphenazine (+amitriptyline)
- Pifluthixol
- Thioridazine
- Thiothixene
- Trifluoperazine (+tranylcypromine)
- Zuclopenthixol
- Atypical antipsychotics: Asenapine
- Clorotepine
- Clotiapine
- Clozapine
- DHA-clozapine
- Fluperlapine
- Iloperidone
- Norclozapine
- Norquetiapine
- Olanzapine (++fluoxetine)
- Paliperidone
- Quetiapine
- Risperidone
- Tefludazine
- Zicronapine
- Ziprasidone
- Zotepine
- Others: Berupipam
- Ecopipam
- EEDQ
- Metitepine (methiothepin)
- Odapipam
- Perlapine
- SCH-23390
|
|---|
|
|---|
| D2-like | | Agonists |
- Adamantanes: Amantadine
- Memantine
- Rimantadine
- Aminotetralins: 5-OH-DPAT
- 7-OH-DPAT
- 8-OH-PBZI
- Rotigotine
- UH-232
- Ergolines: Bromocriptine
- Cabergoline
- Dihydroergocryptine
- Epicriptine
- Ergocornine
- Lergotrile
- Lisuride
- LSD
- Pergolide
- Terguride
- Dihydrexidine derivatives: 2-OH-NPA
- Ciladopa
- Dihydrexidine
- Dinoxyline
- N,N-Propyldihydrexidine
- Phenethylamines: Deoxyepinephrine (N-methyldopamine, epinine)
- Dopexamine
- Etilevodopa
- Ibopamine
- L-DOPA (levodopa)
- L-Phenylalanine
- L-Tyrosine
- Melevodopa
- XP21279
- Atypical antipsychotics: Alentemol (U-66444B)
- Aripiprazole (+sertraline)
- Aripiprazole lauroxil
- Bifeprunox
- Brexpiprazole
- Brilaroxazine
- Cariprazine
- F-15063
- Lumateperone
- Norclozapine
- Others: 3-PPP
- A-412997
- ABT-670
- ABT-724
- Adrafinil
- Aplindore
- Apomorphine
- Arketamine
- Armodafinil
- BP-897
- Captodiame
- CP-226,269
- Dizocilpine
- Esketamine
- Flibanserin
- GSK-789,472
- Ketamine
- Mesulergine
- Modafinil
- OSU-6162
- Pardoprunox
- PD-128,907
- PD-168,077
- PF-219,061
- PF-592,379
- Phencyclidine
- Piribedil
- Pramipexole
- Preclamol
- Propylnorapomorphine
- Pukateine
- Quinagolide
- Quinelorane
- Quinpirole
- RDS-127
- Ro10-5824
- Ropinirole
- Roxindole
- Salvinorin A
- SKF-83,959
- Sumanirole
- Talipexole
- Umespirone
- WAY-100,635
|
|---|
| Antagonists | |
|---|
|
|---|
- See also: Receptor/signaling modulators
- Adrenergics
- Serotonergics
- Monoamine reuptake inhibitors
- Monoamine releasing agents
- Monoamine metabolism modulators
- Monoamine neurotoxins
|
 | Original source: https://en.wikipedia.org/wiki/1-(3-Chlorophenyl)-4-(2-phenylethyl)piperazine. Read more |
 From Handwiki
From Handwiki 


 KSF
KSF