Nonene
 From Handwiki
From Handwiki Nonene is an alkene with the molecular formula C9H18. Many structural isomers are possible, depending on the location of the C=C double bond and the branching of the other parts of the molecule. Industrially, the most important nonenes are trimers of propene: Tripropylene. This mixture of branched nonenes is used in the alkylation of phenol to produce nonylphenol, a precursor to detergents, which are also controversial pollutants.[1]
Linear nonenes
| Linear Nonene | ||||
| Name | 1-Nonene | 2-Nonene | 3-Nonene | 4-Nonene |
| Systematic name | Non-1-ene | Non-2-ene | Non-3-ene | Non-4-ene |
| Structure | 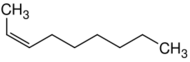 |
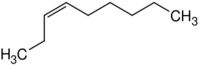 |
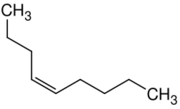 | |
| CAS Number | 124-11-8 |
|
|
|
| 27215-95-8 (all isomers) | ||||
| PubChem | CID 31285 from PubChem | CID 33744 from PubChem | CID 88350 from PubChem | CID 94226 from PubChem |
| Chemical formula | C9H18 | |||
| Molecular weight | 126.24 g·mol−1 | |||
| Melting point | −81 °C[2] | |||
| Boiling point | 147 °C[2] | 144–145 °C[3] | 147 °C[4] | |
| Density | 0,73 g·cm−3 (20 °C)[2] | 0,734 g·cm−3 (25 °C)[3] | 0,734 g·cm−3 (25 °C)[5] | 0,73 g·cm−3[4] |
| GHS hazard pictograms |    [2] [2]
|
 [3] [3]
|
 [5] [5]
|
  [4] [4]
|
| GHS hazard statements | H226, H304, H315, H319, H335 | H226 | H226 | H226, H304 |
| P261, P301+310, P305+351+338, P331 | P210, P233, P240, P241, P242, P243, P280, P301, P310 P331, P303+361+353, P370+378, P403+235, P405, P501 | |||
References
- ↑ Fiege, H.; Voges, H. W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H. J. et al. (2000-06-15) (in en). Phenol Derivatives. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA. pp. a19_313. doi:10.1002/14356007.a19_313. ISBN 978-3-527-30673-2. OCLC 46878292. https://onlinelibrary.wiley.com/doi/10.1002/14356007.a19_313. Retrieved 2022-06-08.
- ↑ 2.0 2.1 2.2 2.3 Record of 1-Nonen in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 1 February 2016.
- ↑ 3.0 3.1 3.2 Sigma-Aldrich Co., trans-2-Nonen, 99%. Retrieved on 2 June 2017.
- ↑ 4.0 4.1 4.2 Entry from 4-Nonene (cis- and trans-mixture) from TCI Europe, retrieved on 2 June 2017
- ↑ 5.0 5.1 Sigma-Aldrich Co., trans-3-Nonene, 99%. Retrieved on 2 June 2017.
 |
Categories: [Alkenes]
↧ Download as ZWI file | Last modified: 10/17/2023 12:32:34 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Nonene | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF