Cephalopoda
 From Britannica 11th Edition (1911)
From Britannica 11th Edition (1911) Cephalopoda, the fifth of the classes into which the zoological phylum Mollusca is divided (see Mollusca). The Cephalopoda are mainly characterized by the concrescence of the foot and head. The foot grows forward on each side so as to surround the mouth, the two upgrowths meeting on the dorsal side of the head—whence the name Cephalopoda. The perioral portion of the foot is drawn out into paired arm-like processes; these may be beset with sheathed tentacles or with suckers or hooks, or both. The epipodia are expanded into a pair of muscular lobes right and left, which are bent round towards one another so that their free margins meet and constitute a short tube—the siphon or funnel. The hind-foot is either very small or absent. A distinctive feature of the Cephalopoda is their bilateral symmetry and the absence of anything like the torsion of the visceral mass seen in the Anisopleurous Gastropoda.
The anus, although it may be a little displaced from the median line, is approximately median and posterior. The mantle-skirt is deeply produced posteriorly, forming a large sub-pallial chamber around the anus. By the side of the anus are placed the single or paired apertures of the nephridia, the genital apertures (paired only in Nautilus, in female Octopoda, female Ommatostrephes and male Eledone), and the paired ctenidia. The visceral hump or dome is elevated, and may be very much elongated in a direction almost at right angles to the primary horizontal axis of the foot.
A shell is frequently, but not invariably, secreted on the visceral hump and mantle-skirt. The shell is usually light in substance or lightened by air-chambers in correlation with the free-swimming habits of the Cephalopoda. It may be external or internal, that is, enclosed in folds of the mantle. Very numerous minute pigmented sacs, capable of expansion and contraction, and known as chromatophores, are usually present in the integument. The sexes are separate.
The ctenidia are well developed as paired gill-plumes, serving as the efficient branchial organs (figs. 4, 24),
The vascular system is very highly developed; the heart consists of a pair of auricles and a ventricle (figs. 12, 28). Branchial hearts are formed on the afferent vessels of the branchiae. It is not known to what extent the minute subdivision of the arteries extends, or whether there is a true capillary system.
The pericardium is extended so as to form a very large sac, passing among the viscera dorsalwards and sometimes containing the ovary or testis—the viscero-pericardial sac—which opens to the exterior either directly or through the renal organs. It has no connexion with the vascular system. The renal organs are always paired sacs, the walls of which invest the branchial afferent vessels (figs. 28, 29). They open each by a pore into the viscero-pericardial sac, except in Nautilus. The anal aperture is median and raised on a papilla. Jaws (fig. 6, e) and a radula (fig. 9) are well developed. The jaws have the form of powerful beaks, either horny or calcified (Nautilus), and are capable of inflicting severe wounds.
Cerebral, pleural and pedal ganglia are present, but the connectives are shortened and the ganglia concentrated and fused in the cephalic region. Large special ganglia (optic, stellate and supra-buccal) are developed. Sense-organs are highly developed; the eye exhibits a very special elaboration of structure in the Dibranchiata, and a remarkable archaic form in the nautilus. Otocysts are present in all. The typical osphradium is not present, except in Nautilus, but other organs are present in the cephalic region, to which an olfactory function is ascribed both in Nautilus and in the other Cephalopoda.
Hermaphroditism is unknown in Cephalopoda; male and female individuals always being differentiated. The genital aperture and duct is sometimes single, when it is the left; sometimes the typical pair is developed right and left of the anus. The males of nearly all Cephalopoda have been shown to be characterized by a peculiar modification of the arm-like processes or lobes of the fore-foot, connected with the copulative function. The term hectocotylization is applied to this modification (see figs. 6, 24). Elaborate spermatophores or sperm-ropes are formed by all Cephalopoda, and very usually the female possesses special capsule-forming and nidamental glands for providing envelopes to the eggs (fig. 4, g.n.). The egg is large, and the development is much modified by the presence of an excessive amount of food-material diffused in the protoplasm of the egg-cell. Trochosphere and veliger stages of development are consequently not recognizable.
The Cephalopoda are divisible into two orders, Tetrabranchiata and Dibranchiata, the names of which (due to Sir R. Owen) describe the number of gill-plumes present; but in fact there are several characters, of as great importance as those derived from the gills, by which the members of these two orders are separated from one another.
Order 1. Tetrabranchiata (= Schizosiphona, Tentaculifera).
Characters.—The inrolled lateral margins of the epipodia are not fused, but form a siphon by apposition (fig. 4). The circumoral lobes of the fore-foot carry numerous retractile tentacles, not suckers (fig. 6). There are two pairs of ctenidial gills (hence Tetrabranchiata), and two pairs of renal organs, consequently four renal apertures (fig. 4). The viscero-pericardial chamber opens by two independent apertures to the exterior, and not into the renal sacs. There are two oviducts (right and left) in the female, and two sperm-ducts in the male, the left duct in both sexes being rudimentary. A large external shell, either coiled or straight, is present, and is not enclosed by reflections of the mantle-skirt. The shell consists of a series of chambers, the last-formed of which is occupied by the body of the animal, the hinder ones (successively deserted) containing gas (fig. 1). The pair of cephalic eyes are hollow chambers (fig. 14. A), opening to the exterior by minute orifices (pin-hole camera), and devoid of refractive structures. A pair of osphradia are present at the base of the gills (fig. 4, olf). Salivary glands are wanting. An ink-sac is not present. Branchial hearts are not developed on the branchial afferent vessels.
 | |
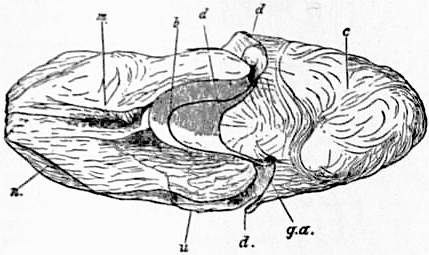
| Fig. 1.—Lateral view of the female Pearly Nautilus, contracted by spirit
and lying in its shell, the right half of which is cut away (from Gegenbaur,
after Owen). | |
|
a, Visceral hump. b, Portion of the free edge of the mantle-skirt reflected on to the shell,—the edge of the mantle-skirt can be traced downwards and forwards around the base of the mid-foot or siphon i. l, l, Superficial origin of the retractor muscle of the mid-foot (siphon), more or less firmly attached to the shell, of which a small piece (s) is seen between the letters l, l. s, (farther back) points to the siphuncular pedicle, which is broken off short and not continued, as in the perfect state, through the whole length of the siphuncle of the shell, also marked s and s’. |
o, points to the right eye. t, is placed near the extremities of the contracted tentacles of the outer or annular lobe of the fore-foot—the jointed tentacles are seen protruding a little from their long cylindrical sheaths. v, The dorsal “hood” formed by an enlargement in this region of the annular lobe of the fore-foot (m in figs. 2, 3). V, A swelling of the mantle-skirt, indicating the position on its inner face of the nidamental gland (see fig. 4, g.n.). |
Visceral Hump and Shell.—The visceral hump of Nautilus (if we exclude from consideration the fine siphuncular pedicle which it trails, as it were, behind it) is very little, if at all, affected by the coiled form of the shell which it carries, since the animal always slips forward in the shell as it grows, and inhabits a chamber which is practically cylindrical (fig. 1). Were the deserted chambers thrown off instead of being accumulated behind the inhabited chamber as a coiled series of air-chambers, we should have a more correct indication in the shell of the extent and form of the animal’s body. Amongst Gastropods it is not very unusual to find the animal slipping forward in its shell as growth advances and leaving an unoccupied chamber in the apex of the shell. This may indeed become shut off from the occupied cavity by a transverse septum, and a series of such septa may be formed, but in no Gastropod are these apical chambers known to contain a gas during the life of the animal in whose shell they occur. A further peculiarity of the nautilus shell and of that of the allied extinct Ammonites, Scaphites, Orthoceras, &c., and of the living Spirula, is that the series of deserted air-chambers is traversed by a cord-like pedicle extending from the centro-dorsal area of the visceral hump to the smallest and first-formed chamber of the series. No structure comparable to this siphuncular pedicle is known in any other Mollusca. The siphuncle does not communicate with the coelomic cavity; it is a simple vascular process of the mantle, whose cavity consists of a venous sinus, and whose wall contains a ramification of the pallial artery. There appears to be no doubt that the deserted chambers of the nautilus shell contain in the healthy living animal a gas which serves to lessen the specific gravity of the whole organism. This gas is said to be of the same composition as the atmosphere, with a larger proportion of nitrogen. With regard to its origin we have only conjectures. Each septum shutting off an air-containing chamber is formed during a period of quiescence, probably after the reproductive act, when the visceral mass of the nautilus may be slightly shrunk, and gas is secreted from the dorsal integument so as to fill up the space previously occupied by the animal. A certain stage is reached in the growth of the animal when no new chambers are formed. The whole process of the loosening of the animal in its chamber and of its slipping forward when a new septum is formed, as well as the mode in which the air-chambers may be used as a hydrostatic apparatus, and the relation to this use, if any, of the siphuncular pedicle, is involved in obscurity, and is the subject of much ingenious speculation. In connexion with the secretion of gas by the animal, besides the parallel cases ranging from the protozoon Arcella to the physoclistic fishes, from the hydroid Siphonophora to the insect-larva Corethra, we have the identical phenomenon observed in the closely allied Sepia when recently hatched. Here, in the pores of the internal rudimentary shell, gas is observable, which has necessarily been liberated by the tissues which secrete the shell, and not derived from any external source (Huxley).
The coiled shell of Nautilus, and of the majority of extinct Tetrabranchiata, is peculiar in its relation to the body of the animal, inasmuch as the curvature of the coil proceeding from the centro-dorsal area is towards the head or forwards, instead of away from the head and backwards as in other discoid coiled shells such as Planorbis; the coil is in fact absolutely reversed in the two cases. Such a shell is said to be exogastric. But in some extinct forms, e.g. Phragmoceras, Cyrtoceras, Ptenoceras, the shell is coiled towards the ventral side, when it is termed endogastric. Amongst the extinct allies of the nautilus (Tetrabranchiata) we find shells of a variety of shapes, open coils such as Scaphites, leading on to perfectly cylindrical shells with chamber succeeding chamber in a straight line (Orthoceras), whence again we may pass to the corkscrew spires formed by the shell of Turrilites. In some extinct genera, e.g. Gomphoceras, among the Nautiloidea the aperture of the shell is contracted and the edge of the aperture is lobed. In these cases the animal was probably able only to protrude its appendages and not its whole head. The ventral part of the aperture corresponding to the funnel is separated from the dorsal part by a constriction. Hence it is possible to distinguish the ventral and dorsal sides of the shell and to decide whether it was exogastric or endogastric. The direction of the coil of the shell cannot be determined by the position of the siphuncle, which traverses the septa centrally, ventrally or dorsally. Contracted shell apertures occur also in Ammonitoidea, the condition reaching an extreme in Morphoceras, where the original aperture is subdivided by the ingrowth of the sides, so that only five small separate apertures remain. Of these the central probably corresponded to the mouth, two lateral to the eyes, and the remaining two to the pedal appendages.
Head, Foot, Mantle-skirt and Sub-pallial Chamber.—In the pearly nautilus the ovoid visceral hump is completely encircled by the free flap of integument known as mantle-skirt (figs. 2, 3, d, e). In the antero-dorsal region this flap is enlarged so as to be reflected a little over the coil of the shell which rests on it. In the postero-ventral region the flap is deepest, forming an extensive sub-pallial chamber, at the entrance of which e is placed in fig. 3. A view of the interior of the sub-pallial chamber, as seen when the mantle-skirt is retroverted and the observer faces in the direction indicated by the reference line passing from e in fig. 3, is given in fig. 4. With this should be compared the similar view of the sub-pallial chamber of the Dibranchiate Sepia. It should be noted as a difference between Nautilus and the Dibranchiates that in the former the nidamental gland (in the female) lies on that surface of the pallial chamber formed by the dependent mantle-flap (fig. 4, g.n.; fig. 1, V), whilst in the latter it lies on the surface formed by the body-wall; in fact in the former the base of the fold forming the mantle-skirt comprises in its area a part of what is unreflected visceral hump in the latter.
 | |
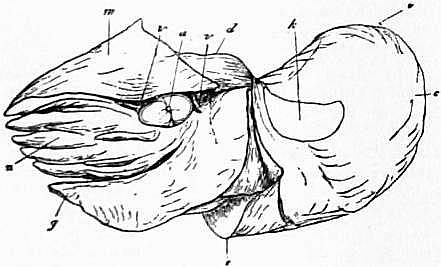
| Fig. 2.—Spirit specimen of female Pearly Nautilus, removed from
its shell, and seen from the antero-dorsal aspect (drawn from nature
by A.G. Bourne). | |
m, The dorsal “hood” formed by the enlargement of the outer or annular lobe of the fore-foot, and corresponding to the sheaths of two tentacles (g, g in fig. 6). n, Tentacular sheaths of lateral portion of the annular lobe. u, The left eye. b, The nuchal plate, continuous at its right and left posterior angles with the root of the mid-foot, and corresponding to the nuchal cartilage of Sepia. |
c, Visceral hump. d, The free margin of the mantle-skirt, the middle letter d points to that portion of the mantle-skirt which is reflected over a part of the shell as seen in fig. 1, b; the cup-like fossa to which b and d point in the present figure is occupied by the coil of the shell. g.a. points to the lateral continuation of the nuchal plate b to join the root of the mid-foot or siphon. |
 | |
| Fig. 3.—Lateral view of the same specimen as that drawn in fig. 2.
Letters as in that figure with the following additions— | |
e, points to the concave margin of the mantle-skirt leading into the sub-pallial chamber. g, The mid-foot or siphon. k, The superficial origin of its retractor muscles closely applied to the shell and serving to hold the animal in its place. |
l, The siphuncular pedicle of the visceral hump broken off short. v, v, The superior and inferior ophthalmic tentacles. |
The apertures of the two pairs of renal sacs, of the viscero-pericardial sac, of the genital ducts, and of the anus, are shown in position on the body-wall of the pallial chamber of Nautilus in figs. 4, 5. There are nine apertures in all, one median (the anus) and four paired. Besides these apertures we notice two pairs of gill-plumes which are undoubtedly typical ctenidia, and a short papilla (the osphradium) between each anterior and posterior gill-plume (see figs. 4, 5, and explanation). As compared with this in a Dibranchiate, we find (fig. 25) only four apertures, viz. the median anus with adjacent orifice of the ink-sac, the single pair of renal apertures, and one asymmetrical genital aperture (on the left side) except in female Octopoda and a few others, where the genital ducts and their apertures are paired. No viscero-pericardial pores are present on the surface of the pallial chamber, since in the Dibranchiata the viscero-pericardial sac opens by a pore into each nephridium instead of directly to the surface. A single pair of ctenidia (gill-plumes) is present instead of the two pairs in Nautilus. The existence of two pairs of ctenidia and of two pairs of renal sacs in Nautilus, placed one behind the other, is highly remarkable. The interest of this arrangement is in relation to the general morphology of the Mollusca, for it is impossible to view this repetition of organs in a linear series as anything else than an instance of metameric segmentation, comparable to the segmentation of the ringed worms and Arthropods. The only other example which we have of this metamerism in the Mollusca is presented by the Chitons. There we find not two pairs of ctenidia merely, but sixteen pairs (in some species more) accompanied by a similar metamerism of the dorsal integument, which carries eight shells. In Chiton the renal organs are not affected by the metamerism as they are in Nautilus. It is impossible on the present occasion to discuss in the way which their importance demands the significance of these two instances among Mollusca of incomplete or partial metamerism; but it would be wrong to pass them by without insisting upon the great importance which the occurrence of these isolated instances of metameric segmentation in a group of otherwise unsegmented organisms possesses, and the light which they may be made to throw upon the nature of metameric segmentation in general.
 | |
| Fig. 4.—View of the postero-ventral surface of a female Pearly
Nautilus, the mantle-skirt (c) being completely reflected so as to
show the inner wall of the sub-pallial chamber (drawn from nature
by A.G. Bourne). | |
a, Muscular band passing from the mid-foot to the integument. b, The valve on the surface of the funnel, partially concealed by the inrolled lateral margin of the latter. c, The mantle-skirt retroverted. an, The median anus. x, Post-anal papilla of unknown significance. g.n., Nidamental gland. r.ov, Aperture of the right oviduct. |
l.ov, Aperture of the rudimentary left oviduct (pyriform sac of Owen). neph.a, Aperture of the left anterior renal sac. neph.p, Aperture of the left posterior renal sac. viscper, Left aperture of the viscero-pericardial sac. olf, The left osphradium placed near the base of the anterior gill-plume. |
| The four gill-plumes (ctenidia) are not lettered. | |
The foot and head of Nautilus are in the adult inextricably grown together, the eye being the only part belonging primarily to the head which projects from the all-embracing foot. The fore-foot or front portion of the foot has the form of a number of lobes carrying tentacles and completely surrounding the mouth (figs. 2, 3). The epipodia incline towards each other posteriorly so as to form an incomplete siphon (fig. 4), a condition which is completed and rendered permanent in the tubular funnel of Dibranchiata. The epipodial nature of the funnel is well seen in young embryos, in which this organ is situated laterally and posteriorly between the mantle and the foot.
 |
| Fig. 5.—View of the postero-ventral surface of a male Pearly Nautilus, the mantle-skirt (c) being completely reflected so as to show the inner wall of the sub-pallial chamber, and the four ctenidia and the foot cut short (drawn from nature by A.G. Bourne). pe, Penis, being the enlarged termination of the right spermatic duct; l.sp, aperture of the rudimentary left spermatic duct (pyriform sac of Owen). Other letters as in fig. 4. |
The lobes of the fore-foot of Nautilus and of the other Cephalopoda require further description. It has been doubted whether these lobes were rightly referred (by T.H. Huxley) to the fore-foot, and it has been maintained by some zoologists (H. Grenadier, H. von Jhering) that they are truly processes of the head. It appears to be impossible to doubt that the lobes in question are the fore-portion of the foot, when their development is examined (see fig. 35), further, when the fact is considered that they are innervated by the pedal ganglion. The fore-foot of Nautilus completely surrounds the buccal cone (fig. 6, e), so as to present an appearance with its expanded tentacles similar to that of the disk of a sea-anemone (Actinia). A.G. Bourne, of University College, prepared from actual specimens the drawings of this part in the male and female Nautilus reproduced in fig. 6, and restored the parts to their natural form when expanded. The drawings show very strikingly the difference between male and female. In the females (lower figure), we observe in the centre of the disk the buccal cone e carrying the beak-like pair of jaws which project from the finely papillate buccal membrane. Three tentaculiferous lobes of the fore-foot are in immediate contact with this buccal cone; they are the right and left (c, c) inner lobes, and the inferior inner lobe (d)—called inferior because it really lies ventralwards of the mouth. This inner inferior lobe is clearly a double one, representing a right and left inner inferior lobe fused into one. A lamellated organ on its surface, known as Owen’s organ, probably olfactory in function (n), marks the separation of the constituent halves of this double lobe. Each half carries a group of fourteen tentacles. The right and the left inner lobes (c, c) each carry twelve tentacles. External to these three lobes the muscular substance of the mouth-embracing foot is raised into a wide ring, which becomes especially thick and large in the dorsal region where it is notably modified in form, offering a concavity into which the coil of the shell is received, and furnishing a protective roof to the retracted mass of tentacles. This part of the external annular lobe of the fore-foot is called the “hood” (figs. 2, 3, m). The median antero-posterior line traversing this hood exactly corresponds to the line of concrescence of the two halves of the fore-foot, which primitively grew forward one on each side of the head, and finally fused together along this line in front of the mouth. The tentacles carried by the great annular lobe are nineteen on each side, thirty-eight in all. They are called “digital,” and are somewhat larger than the “labial” tentacles carried on the three inner lobes. The dorsalmost pair of tentacles (marked g in fig. 6) are the only ones which actually belong to that part of the disk which forms the great dorsal hood m. The hood is, in fact, to a large extent formed by the enlarged sheaths of these two tentacles. All the tentacles of the circumoral disk are set in remarkable tubular sheaths, into which they can be drawn. The sheaths of some of those belonging to the external or annular lobe are seen in fig. 3, marked n. The sheaths are muscular as well as the tentacles, and are simply tubes from the base of which the solid tentacle grows. The functional significance of this sheathing arrangement is as obscure as its morphological origin. With reference to the latter, it appears highly probable that the tubular sheath represents the cup of a sucker such as is found on the fore-foot of the Dibranchiata. In any case, it seems to the writer impossible to doubt that each tentacle, and its sheath on a lobe of the circumoral disk of Nautilus, corresponds to a sucker on such a lobe of a Dibranchiate. W. Keferstein follows Sir R. Owen in strongly opposing this identification, and in regarding such tentacle as the equivalent of a whole lobe or arm of a Decapod or Octopod Dibranch. The details of these structures, especially in the facts concerning the hectocotylus and spadix, afford the most conclusive reasons for dissenting from Owen’s view. On the ventral side an extensive part of the internal surface of the muscular ring is laminated, forming the so-called “organ of Valenciennes,” peculiar to the female and serving for the attachment of the spermatophores. We have so far enumerated in the female nautilus ninety tentacles. Four more remain which have a very peculiar position, and almost lead to the suggestion that the eye itself is a modified tentacle. These remaining tentacles are placed one above (before) and one below (behind) each eye, and bring up the total to ninety-four (fig. 3 v, v).
 | |
| Fig. 6.—Male (upper) and female (lower) specimens of Nautilus
pompilius as seen in the expanded condition, the observer looking
down on to the buccal cone e; one-third the natural size linear. The
drawings have been made from actual specimens by A.G. Bourne,
B. Sc., University College, London. | |
a, The shell. b, The outer ring-like expansion (annular lobe) of the circumoral muscular mass of the fore-foot, carrying nineteen tentacles on each side—posteriorly this is enlarged to form the “hood” (marked v in fig. 1 and m in figs. 2 and 3). giving off the pair of tentacles marked g in the present figure. c, The right and left inner lobes of the fore-foot, each carrying twelve tentacles in the female, in the male subdivided into p, the “spadix” or hectocotylus on the left side, and q, the “anti-spadix,” a group of four tentacles on the right side—it is thus seen that the subdivided right and left inner lobes of the male correspond to the undivided right and left inner lobes of the female. d, The inner inferior lobe of the fore-foot, a bilateral structure in the female carrying two groups, each of fourteen tentacles, separated from one another by a lamellated organ n, supposed to be olfactory in function—in the male the inner inferior lobe of the fore-foot is very much reduced, and has the form of a paired group of lamellae (d in the upper figure). e, The buccal cone, rising from the centre of the three inner lobes, and fringing the protruded calcareous beaks or jaws with a series of minute papillae. |
f, The tentacles of the outer circumoral lobe or annular lobe of the fore-foot projecting from their sheaths. g, The two most posterior tentacles of this series belonging to that part of the annular lobe which forms the hood (m in figs. 2 and 3). i, Superior ophthalmic tentacle. k, Inferior ophthalmic tentacle. l, Eye. m, Paired laminated organ on each side of the base of the inner inferior lobe (d) of the female. n, Olfactory lamellae upon the inner inferior lobe (in the female). o, The siphon (mid-foot). p, The spadix (in the male), the hectocotylized portion of the left inner lobe of the fore-foot representing four modified tentacles, eight being left unmodified. q, The anti-spadix (in the male), being four of the twelve tentacles of the right inner lobe of the fore-foot isolated from the remaining eight, and representing on the right side the differentiated spadix of the left side. The four tentacles of the anti-spadix are set, three on one base and one on a separate base. |
In the adult male nautilus we find the following important differences in the tentaculiferous disk as compared with the female (see upper drawing in fig. 6). The inner inferior lobe is rudimentary, and carries no tentacles. It is represented by three groups of lamellae (d), which are not fully exposed in the drawing. The right and left inner lobes are subdivided each into two portions. The right shows a larger portion carrying eight tentacles, and smaller detached groups (q) of four tentacles, of which three have their sheaths united whilst one stands alone. These four tentacles may be called the “anti-spadix.” The left inner lobe shows a similar larger portion carrying eight tentacles, and a curious conical body behind it corresponding to the anti-spadix. This is the “spadix.” It carries no tentacles, but is terminated by imbricated lamellae. These lamellae appear to represent the four tentacles of the anti-spadix of the right internal lobe, and are generally regarded as corresponding to that modification of the sucker-bearing arms of male Dibranchiate Siphonopods to which the name “hectocotylus” is applied. The spadix is in fact the hectocotylized portion of the fore-foot of the male nautilus. The hectocotylized arm or lobe of male Dibranchiata is connected with the process of copulation, and in the male nautilus the spadix has probably a similar significance, though it is not possible to suggest how it acts in this relation. It is important to observe that the modification of the fore-foot in the male as compared with the female nautilus is not confined to the existence of the spadix. The anti-spadix and the reduction of the inner inferior lobe are also male peculiarities. The external annular lobe in the male does not differ from that of the female; it carries nineteen tentacles on each side. The four ophthalmic tentacles are also present. Thus in the male nautilus we find altogether sixty-two tentacles, the thirty-two additional tentacles of the female being represented by lamelliform structures.
Musculature, Fins and, Cartilaginous Skeleton.—Without entering into a detailed account of the musculature of Nautilus, we may point out that the great muscular masses of the fore-foot and of the mid-foot (siphon) are ultimately traceable to a large transverse mass of muscular tissue, the ends of which are visible through the integument on the right and left surfaces of the body dorsal of the free flap of the mantle-skirt (fig. 1, l, l, and fig. 3, k). These muscular areae have a certain adhesion to the shell, and serve both to hold the animal in its shell and as the fixed supports for the various movements of the tentaculiferous lobes and the siphon. They are to be identified with the ring-like area of adhesion by which the foot-muscle of the limpet is attached to the shell of that animal. In the Dibranchs a similar origin of the muscular masses of the fore-foot and mid-foot from the sides of the shell—modified, as this is, in position and relations—can be traced.
 |
|
Fig. 7.—Minute structure of the
cartilage of Loligo (from Gegenbaur,
after Furbringer) a, Simple cells. b, Dividing cells. c, Canaliculi. d, An empty cartilage capsule with its pores. e, Canaliculi in section. |
In Nautilus there are no fin-like expansions of the integument, whereas such occur in the Decapod Dibranchs along the sides of the visceral hump (figs. 15, 16). As an exception among Octopoda lateral fins occur in Pinnoctopus (fig. 38, A), and in Cirrhoteuthis (fig. 38, D).
In Nautilus there is a curious plate-like expansion of integument in the mid-dorsal region just behind the hood, lying between that structure and the portion of mantle-skirt which is reflected over the shell. This is shown in fig. 2, b. If we trace out the margin of this plate we find that it becomes continuous on each side with the sides of the funnel. In Sepia and other Decapods (not in Octopods) a closely similar plate exists in an exactly corresponding position (see b in figs. 10, 26). In Sepia a cartilaginous development occurs here immediately below the integument forming the so-called “nuchal plate,” drawn in fig. 8, D. The morphological significance of this nuchal lamella, as seen both in Nautilus and in Sepia, is not obvious. Cartilage having the structure shown in fig. 7 occurs in various regions of the body of Cephalopoda. In all Glossophorous Mollusca the lingual apparatus is supported by internal skeletal pieces, having the character of cartilage; but in the Cephalopoda such cartilage has a wider range.
In Nautilus a large H-shaped piece of cartilage is found, forming the axis of the funnel (fig. 8, A, B). Its hinder part extends up into the head and supports the peri-oesophageal nerve-mass (a), whilst its two anterior rami extend into the tongue-like siphon. In Sepia, and Dibranchs generally, the cartilage takes a different form, as shown in fig. 8, C. The processes of this cartilage cannot be identified in any way with those of the capito-pedal cartilage of Nautilus. The lower larger portion of this cartilage in Sepia is called the cephalic cartilage, and forms a complete ring round the oesophagus; it completely invests also the ganglionic nerve-collar, so that all the nerves from the latter have to pass through foramina in the cartilage. The outer angles of this cartilage spread out on each side so as to form a cup-like receptacle for the eyes. The two processes springing right and left from this large cartilage in the median line (fig. 8, C) are the “pre-orbital cartilages”; in front of these, again, there is seen a piece like an inverted T, which forms a support to the base of the “arms” of the fore-foot, and is the “basi-brachial” cartilage. The Decapod Dibranchs have, further, the “nuchal cartilage” already mentioned, and in Sepia, a thin plate-like “sub-ostracal” or (so-called) dorsal cartilage, the anterior end of which rests on and fits into the concave nuchal cartilage. In Octopoda there is no nuchal cartilage, but two band-like “dorsal cartilages.” In Decapods there are also two cartilaginous sockets on the sides of the funnel—“siphon-hinge cartilages”—into which fleshy knobs of the mantle-skirt are loosely fitted. In Sepia, along the whole base-line of each lateral fin of the mantle (fig. 15), is a “basi-pterygial cartilage.” It is worthy of remark that we have, thus developed, in Dibranch Cephalopods a more complete internal cartilaginous skeleton than is to be found in some of the lower vertebrates. There are other instances of cartilaginous endo-skeleton in groups other than the Vertebrata. Thus in some capito-branchiate Chaetopods cartilage forms a skeletal support for the gill-plumes, whilst in the Arachnids (Mygale, Scorpio) and in Limulus a large internal cartilaginous plate—the ento-sternite—is developed as a support for a large series of muscles.
 | |
| Fig. 8.—Cartilaginous skeleton of Cephalopoda (after Keferstein.) | |
A, Capito-pedal cartilage of Nautilus pompilius. a points to the ridge which supports the pedal portion of the nerve-centre. |
B, Lateral view of the same—the large anterior processes are sunk in the muscular substance of the siphon. C, Cephalic cartilages of Sepia officinalis. D, Nuchal cartilage of Sepia officinalis. |
Alimentary Tract.—The buccal cone of Nautilus is terminated by a villous margin (buccal membrane), surrounding the pair of beak-like jaws, of which the ventral projects over the dorsal. These are very strong and dense in Nautilus, being calcified. Fossilized beaks of Tetrabranchiata are known under the name of rhyncholites. In Dibranchs the beaks are horny, but similar in shape to those of Nautilus. They resemble in general those of a parrot, the lower beak being the larger and overlapping the upper or dorsal beak. The lingual ribbon and odontophoral apparatus have the structure which is typical for Glossophorous Mollusca. In fig. 9, A is represented a single row of teeth from the lingual ribbon of Nautilus, and in fig. 9, B, C, of other Cephalopoda.
In Nautilus a long and wide crop or dilated oesophagus (fig. 10, cr) passes from the muscular buccal mass, and at the apex of the visceral hump passes into a highly muscular stomach, resembling the gizzard of a bird (fig 10, gizz). A nearly straight intestine passes from the muscular stomach to the anus, near which it develops a small caecum. In other Cephalopods the oesophagus is usually narrower and the muscular stomach more capacious, whilst a very important feature in the alimentary tract is formed by the caecum. In all but Nautilus the caecum lies near the stomach, and may be very capacious—much larger than the stomach in Loligo vulgaris—or elongated into a spiral coil. The simple U-shaped flexure of the alimentary tract, as seen in fig. 10, is the only important one which it exhibits in the Cephalopoda. The acini of the large liver of Nautilus are compacted into a solid reddish-brown mass by a firm membrane, as also is the case in the Dibranchiata. The liver has four paired lobes in Nautilus, which open by two bile-ducts into the alimentary canal at the commencement of the intestine. The bile-ducts unite before entering the intestine. In Dibranchiata the two large lobes of the liver are placed antero-dorsally (beneath the shell in Decapoda), and the bile-ducts open into the caecum. Upon the bile-ducts in Dibranchiata are developed yellowish glandular diverticula, which are known as “pancreas,” though neither physiologically nor morphologically is there any ground for considering either the so-called liver or the so-called pancreas as strictly equivalent to the glands so denominated in the Vertebrata. In Nautilus the equivalents of the pancreatic diverticula of the Dibranchs can be traced upon the relatively shorter bile-ducts.
 |
| Fig. 9.—Lingual dentition of Cephalopoda. A, A single row of lingual teeth of Nautilus pompilius (after Keferstein). B, Two rows of lingual teeth of Sepia officinalis (after Troschel). C, Lingual teeth of Eledone cirrhosa (after Loven). |
 | |
| Fig. 10.—Diagram representing a vertical approximately median
antero-posterior section of Nautilus pompilius (from a drawing by
A.G. Bourne). The parts which are quite black are the cut muscular
surfaces of the foot and buccal mass. | |
a, The shell. b, The nuchal plate, identical with the nuchal cartilage of Sepia (see fig. 2, b). c, The integument covering the visceral hump. d, The mantle flap or skirt in the dorsal region where it rests against the coil of the shell. e, The inferior margin of the mantle-skirt resting on the lip of the shell represented by the dotted line. f, The pallial chamber with two of the four gills. g, The vertically cut median portion of the mid-foot (siphon). h, The capito-pedal cartilage (see fig. 8). i, The valve of the siphon. l, The siphuncular pedicle (cut short). m, The hood or dorsal enlargement of the annular lobe of the fore-foot. n, Tentacles of the annular lobe. p, Tentacles of inner inferior lobe. |
q, Buccal membrane. r, Upper jaw or beak. s, Lower jaw or beak. t, Lingual ribbon. x, The viscero-pericardial sac. n.c, Nerve-collar. oe, Oesophagus. cr, Crop. gizz, Gizzard. int, Intestine. an, Anus. nept, Aperture of a nephridial sac. r.e, Renal glandular masses on the walls of the afferent branchial veins (see fig. 11). a.b.v, Afferent branchial vessel. e.b.v, Efferent branchial vessel. vt, Ventricle of the heart. |
Posterior salivary glands are not developed in Nautilus, but on each side in the wall of the buccal mass is a gland corresponding to the anterior salivary gland of the Dibranchiata. No ink-sac is present in Nautilus.
 | |
| Fig. 11.—Diagram to show the relations of the four nephridial
sacs, the viscero-pericardial sac, and the heart and large vessels in
Nautilus (drawn by A.G. Bourne). | |
neph, neph, on the right side point to the two nephridia of that side (the two of the opposite side are not lettered)—each is seen to have an independent aperture. x is the viscero-pericardial sac, the dotted line indicating its backward extension. visc. per. apert, marks an arrow introduced into the right aperture of the viscero-pericardial sac. |
r.e, r.e, point to the glandular enlarged walls of the afferent branchial vessels—two small glandular bodies of the kind are seen to project into each nephridial sac, whilst a larger body of the same kind depends from each of the four branchial afferent vessels into the viscero-pericardial sac. v.c, Vena-cava. vent, Ventricle of the heart. ao, Cephalic aorta (the small abdominal aorta not drawn). a.b.v, Branchial vessel. e.v.b, Efferent branchial vessel. |
Coelom, Blood-vascular System and Excretory Organs.—Nautilus and the other Cephalopoda conform to the general Molluscan characters in regard to these organs. Whilst the general visceral cavity forms a lacunar blood-system or series of narrow spaces, connected with the trunks of a well-developed vascular system, that part of the original coelom surrounding the heart and known as the Molluscan pericardium is shut off from this general blood-lymph system, and communicates, directly in Nautilus, in the rest through the renal sacs, with the exterior. In the Cephalopoda this specialized pericardial cavity is particularly large, and has been recognized as distinct from the blood-carrying spaces, even by anatomists who have not considered the pericardial space of other Mollusca to be thus isolated. The enlarged pericardium, which may even take the form of a pair of sacs, has been variously named, but is best known as the viscero-pericardial sac or chamber. In Nautilus this sac occupies the whole of the postero-dorsal surface and a part of the antero-dorsal (see fig. 10, x), investing the genital and other viscera which lie below it, and having the ventricle of the heart suspended in it. Certain membranes forming incomplete septa, and a curious muscular band—the pallio-cardiac band—traverse the sac. The four branchial afferent veins, which in traversing the walls of the four renal sacs give off, as it were, glandular diverticula into those sacs, also give off at the same points four much larger glandular masses, which hang freely into the viscero-pericardial chamber (fig. 11, r.e). In Nautilus the viscero-pericardial sac opens to the exterior directly by a pair of apertures, one placed close to the right and one close to the left posterior renal aperture (fig. 5, visc. per). This direct opening of the pericardial sac to the exterior is an exception to what occurs in all other Mollusca. In all other Molluscs the pericardial sac opens into the renal organs, and through them or the one renal organ to the exterior. In Nautilus there is no opening from the viscero-pericardial sac into the renal sacs. Therefore the external pore of the viscero-pericardial sac may possibly be regarded as a shifting of the reno-pericardial orifice from the actual wall of the renal sac to a position alongside of its orifice. Parallel cases of such shifting are seen in the varying position of the orifice of the ink-bag in Dibranchiata, and in the orifice of the genital ducts of Mollusca, which in some few cases (e.g. Spondylus) open into the renal organs, whilst in other cases they open close by the side of the renal organs on the surface of the body. The viscero-pericardial sac of the Dibranchs is very large also, and extends into the dorsal region. It varies in shape—that is to say, in the extensions of its area right and left between the various viscera—in different genera, but in the Decapods is largest. In an extension of this chamber is placed the ovary of Sepia, whilst the ventricle of the heart and the branchial hearts and their appendages also lie in it. It is probable that water is drawn into this chamber through the renal sacs, since sand and other foreign matters are found in it. In all it opens into the pair of renal sacs by an orifice on the wall of each, not far from the external orifice (fig. 29, y, y′). There does not seem any room for doubting that each orifice corresponds to the reno-pericardial orifice which we have seen in the Gastropoda, and shall find again in the Lamellibranchia.
 | |
| Fig. 12.—Diagram to show the relations of the heart in the Mollusca. (From Gegenbaur.) | |
A, Part of the dorsal vascular trunk and transverse trunks of a worm. B, Ventricle and auricles of Nautilus. C, Of a Lamellibranch, of Chiton, or of Loligo. D, Of Octopus. |
E, Of a Gastropod. a, Auricle. v, Ventricle. ac, Arteria = cephalica = (aorta). ai, Arteria abdominalis. The arrows show the direction of the blood-current. |
The circulatory organs, blood-vessels and blood of Nautilus do not differ greatly from those of Gastropoda. The ventricle of the heart is a four-cornered body, receiving a dilated branchial efferent vessel (auricle) at each corner (fig. 11). It gives off a cephalic aorta anteriorly, and a smaller abdominal aorta posteriorly. The diagram, fig. 12, serves to show how this simple form of heart is related to the dorsal vessel of a worm or of an Arthropod, and how by a simple flexure of the ventricle (D) and a subsequent suppression of one auricle, following on the suppression of one branchia, one may obtain the form of heart characteristic of the anisopleurous Gastropoda (excepting the Aspidobranchia). The flexed condition of the heart is seen in Octopus, and is to some extent approached by Nautilus, the median vessels not presenting that perfect parallelism which is shown in the figure (B). The most remarkable feature presented by the heart of Nautilus is the possession of four instead of two auricles, a feature which is simply related to the metamerism of the branchiae. By the left side of the heart of Nautilus, attached to it by a membrane, and hanging loosely in the viscero-pericardial chamber, is the pyriform sac of Owen. This has been shown to be the rudimentary left oviduct or sperm-duct, as the case may be (E.R. Lankester and A.G. Bourne), the functional right ovi-sac and its duct being attached by a membrane to the opposite side of the heart.
The cephalic and abdominal aortae of Nautilus appear, after running to the anterior and posterior extremes of the animal respectively, to open into sinus-like spaces surrounding the viscera, muscular masses, &c. These spaces are not large, but confined and shallow. Capillaries are stated to occur in the integument. In the Dibranchs the arterial system is very much more complete; it appears in some cases to end in irregular lacunae or sinuses, in other cases in true capillaries which lead on into veins. An investigation of these capillaries in the light of modern histological knowledge is much needed. From the sinuses and capillaries the veins take origin, collecting into a large median trunk (the vena cava), which in the Dibranchs as well as in Nautilus has a ventral (postero-ventral) position, and runs parallel to the long axis of the animal. In Nautilus this vena cava gives off at the level of the gills four branchial afferent veins (fig. 11, v.c.), which pass into the four gills without dilating. In the Dibranchs at a similar position the vena cava gives off a right and a left branchial afferent vein, each of which, traversing the wall of the corresponding renal sac and receiving additional factors, dilates at the base of the corresponding branchial plume, forming there a pulsating sac—the branchial heart. Attached to each branchial heart is a curious glandular body, which may possibly be related to the larger masses (fig. 11, r.e.) which depend into the viscero-pericardial cavity from the branchial afferent veins of Nautilus. From the dilated branchial heart the branchial afferent vessel proceeds, running up the adpallial face of the gill-plume. From each gill-plume the blood passes by the branchial efferent vessels to the heart, the two auricles being formed by the dilatation of these vessels.
The blood contains the usual amoeboid corpuscles, and a diffused colouring matter—the haemocyanin of Fredericque—which has been found also in the blood of Helix, and in that of the Arthropods Homarus and Limulus. It is colourless in the oxidized, blue in the deoxidized state, and contains copper as a chemical constituent.
The renal sacs and renal glandular tissue are closely connected with the branchial advehent vessels in Nautilus and in the other Cephalopoda. The arrangement is such as to render the typical relations and form of a renal tube difficult to trace. In accordance with the metamerism of Nautilus already noticed, there are two pairs of renal organs. Each assumes the form of a sac opening by a pore to the exterior. As is usual in renal tubes a glandular and a non-glandular portion are distinguished in each sac; these portions, however, are not successive parts of a tube, as happens in other cases, but they are localized areae of the wall of the sac. The glandular renal tissue is, in fact, confined to a tract extending along that part of the sac’s wall which immediately invests the great branchial afferent vein. The vein in this region gives off directly from its wall a complete herbage of little venules, which branch and anastomose with one another, and are clothed by the glandular epithelium of the renal sac. The secretion is accumulated in the sac and passed by its aperture to the exterior. Probably the nitrogenous excretory product is very rapidly discharged; in Nautilus a pink-coloured powder is found accumulated in the renal sacs, consisting of calcium phosphate. The presence of this phosphatic calculus by no means proves that such was the sole excretion of the renal glandular tissue. In Nautilus a glandular growth like that rising from the wall of the branchial vessel into its corresponding renal sac, but larger in size, depends from each branchial afferent vessel into the viscero-pericardial sac and forms the pericardial gland—probably identical with the “appendage” of the branchial hearts of Dibranchs.
The chief difference, other than that of number, between the renal organs of the Dibranchs and those of Nautilus, is the absence of the accessory growths depending into the viscero-pericardial space just mentioned, and, of more importance, the presence in the former of a pore leading from the renal sac into the viscero-pericardial sac (y, y′ in fig. 29). The external orifices of the renal organs are also more prominent in Dibranchs than in Nautilus, being raised on papillae (np in fig. 29; r in fig. 25). In Sepia the two renal sacs give off each a diverticulum dorsalwards, which unites with its fellow and forms a great median renal chamber, lying between the ventral portions of the renal organs and the viscero-pericardial chamber. In Loligo the fusion of the two renal organs to form one sac is still more obvious, since the ventral portions are united. In Octopus the renal sacs are quite separate.
Gonads and Genital Ducts.—In Nautilusit has been shown by E. Ray Lankester and A.G. Bourne that the genital ducts of both sexes are paired right and left, the left duct being rudimentary and forming the “pyriform appendage,” described by Sir R. Owen as adhering by membranous attachment to the ventricle of the heart, and shown by W. Keferstein to communicate by a pore with the exterior. The ovary (female gonad) or the testis (male gonad) lies in Nautilus, as in the Dibranchs, in a distinct cavity walled off from the other viscera, near the centro-dorsal region. This chamber is formed by the coelomic or peritoneal wall; the space enclosed is originally part of the coelom, and in Sepia and Loligo is, in the adult, part of the viscero-pericardial chamber. In Octopus it is this genital chamber which communicates by a right and a left canal with the renal sac, and is the only representative of pericardium. The ovary or testis is itself a growth from the inner wall of this chamber, which it only partly fills. In Nautilus the right genital duct, which is functional, is a simple continuation to the pore on the postero-dorsal surface of the membranous walls of the capsule in which lies the ovary or the testis, as the case may be. The gonad itself appears to represent a single median or bilateral organ.
The ovary forms a large projection into the genital coelom, and the coelomic epithelium is deeply invaginated into the mass of the gonad, so as to constitute an ovarian cavity communicating with the coelom by a narrow aperture. The ova originate in the epithelium, migrate below it and then, as they enlarge, project into the ovarian cavity, pushing the epithelium before them. Each ovum is surrounded by a follicular epithelium which is nourished by numerous blood-vessels, and which penetrates into the surface of the ovum in numerous folds. When mature, the ovum is contained in a membrane or chorion with a micropyle, and escapes by dehiscence of the follicle into the genital coelom and duct. In its passage to the exterior the ovum passes a glandular structure on the wall of the genital capsule, which probably secretes the gelatinous substance enclosing the eggs. In addition to this internal gland there are other accessory glands, which are not related to the genital duct or sac but are differentiations of the wall of the pallial cavity, and occur on the inner wall of the pallium in Nautilus, on the somatic wall in Dibranchiata. In Nautilus they form a continuous mass. These produce the external envelopes of the eggs.
In the male the testis is a specialized portion of the wall of the genital coelom, and has a structure comparable to that of the ovary. The spermatozoa pass through an orifice from the cavity of the testis to the genital capsule, and thence to the spermiduct. The spermiduct is provided with a glandular pouch, and opens into a terminal reservoir known as Needham’s sac or the spermatophore sac. The function of this pouch is to form the spermatophore, which is an elastic tube formed of structureless secretion and invaginated into itself. The deeper part contains the spermatozoa, the external part is called the connective, and is usually much contracted and spirally coiled. When the spermatophore is expelled into the water the connective is extended and evaginated, and the sac containing the sperms bursts. In Nautilus the spermatophore when uncoiled is a little over 30 mm. in length. These spermatophores are somewhat similar to those formed in certain pulmonate Gastropods.
The eggs are laid shortly after copulation. In Nautilus they are laid separately, each being about 4 cm. long and contained in two thick shells, the outer of which is partly open.
 |
|
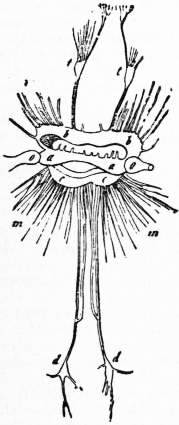
Fig. 13.—Nervous
system of Nautilus pompilius
(from Genebaur,
after Owen). t, t, Ganglion-like enlargements on nerves passing from the pedal ganglion to the inner series of tentacles. t′, Nerves to the tentacles of the outer or annular lobe. b, Pedal ganglion-pair a, Cerebral ganglion-pair. c, Pleuro-visceral ganglionic band (fused pleural and visceral ganglion-pairs). d, Genital ganglion placed on the course of the large visceral nerve, just before it gives off its branchial and its osphradial branches. m, Nerves from the pleural ganglion to the mantle-skirt. |
Nervous System.—Nautilus, like the other Cephalopoda, exhibits a great concentration of the typical Molluscan ganglia, as shown in fig. 13. The ganglia take on a band-like form, and are but little differentiated from their commissures and connectives—an archaic condition reminding us of Chiton. The special optic outgrowth of the cerebral ganglion, the optical ganglion (fig. 13, o), is characteristic. The cerebral ganglion-pair (a) lying above the oesophagus is connected with two suboesophageal ganglion-pairs, of band-like form. The anterior of these is the pedal b, b, and supplies the circumoral lobes and tentacles, and the funnel, a fact which proves the pedal origin of these organs. The hinder band is the visceral and pleural pair fused; from its pleural portion nerves pass to the mantle, from its visceral portion nerves to the branchiae and genital ganglion (fig. 13, d), and in immediate connexion with the latter is a nerve to the osphradium or olfactory papilla. A labial commissure arises by a double root from the cerebral ganglia and gives off a stomatogastric commissure, which passes under the pharynx immediately behind the radula and bears a buccal ganglion on either side.
Special Sense-Organs.—Nautilus possesses a pair of osphradial papillae (fig. 4, olf) corresponding in position and innervation to Spengel’s organ placed at the base of the ctenidia (branchiae) in all classes of Mollusca. This organ has not been detected in other Cephalopoda. Nautilus possesses other olfactory organs in the region of the head. Just below the eye is a small triangular process (not seen in our figures), having the structure of a shortened and highly-modified tentacle and sheath. By A. Valenciennes, who is followed by W. Keferstein, this is regarded as an olfactory organ. The large nerve which runs to this organ originates from the point of juncture of the pedal with the optic ganglion. The lamelliform organ upon the inner inferior tentacular lobe of Nautilus is possibly also olfactory in function. In Dibranchs behind the eye is a pit or open canal supplied by a nerve corresponding in origin to the olfactory nerve of Nautilus above mentioned. Possibly the sense of taste resides in certain processes within the mouth of Nautilus and other Cephalopoda.
The otocysts of Nautilus were discovered by J.D. Macdonald. Each lies at the side of the head, ventral to the eye, resting on the capito-pedal cartilage, and supported by the large auditory nerve which apparently arises from the pedal ganglion but originates in the cerebral. It has the form of a small sac, 1 to 2 mm. in diameter, and contains whetstone-shaped crystals, such as are known to form the otoliths of other Mollusca.
The eye of Nautilus is among the most interesting structures of that remarkable animal. No other animal which has the same bulk and general elaboration of organization has so simple an eye as that of Nautilus. When looked at from the surface no metallic lustre, no transparent coverings, are presented by it. It is simply a slightly projecting hemispherical box like a kettle-drum, half an inch in diameter, its surface looking like that of the surrounding integument, whilst in the middle of the drum-membrane is a minute hole (fig. 3, u). Sir R. Owen very naturally thought that some membrane had covered this hole in life, and had been ruptured in the specimen studied by him. It, however, appears from the researches of V. Hensen that the hole is a normal aperture leading into the globe of the eye, which is accordingly filled by sea-water during life. There is no dioptric apparatus in Nautilus, and in place of refracting lens and cornea we have actually here an arrangement for forming an image on the principle of “the pin-hole camera.” There is no other eye known in the whole animal kingdom which is so constructed. The wall of the eye-globe is tough, and the cavity is lined solely by the naked retina, which is bathed by sea-water on one surface and receives the fibres of the optic nerve on the other (see fig. 14, A). As in other Cephalopods (e.g. fig. 33, Ri, Re, p), the retina consists of two layers of cells, separated by a layer of dark pigment. The most interesting consideration connected with this eye of Nautilus is found when the further facts are noted—(1) that the elaborate lens-bearing eyes of Dibranchiata pass through a stage of development in which they have the same structure as the eye of Nautilus—namely, are open sacs (fig. 34); and (2) that amongst other Mollusca examples of cephalic eyes can be found which in the adult condition are, like the eye of Nautilus and the developing eye of Dibranchs, simple pits of the integument, the cells of which are surrounded by pigment and connected with the filaments of an optic nerve. Such is the structure of the eye of the limpet (Patella), and in such a simple eye we obtain the clearest demonstration of the fact that the retina of the Molluscan cephalic eye, like that of the Arthropod cephalic eye and unlike that of the vertebrate myelonic eye, is essentially a modified area of the general epiderm, and that the sensitiveness of its cells to the action of light and their relation to nerve-filaments is only a specialization and intensifying of a property common to the whole epiderm of the surface of the body. What, however, strikes us as especially remarkable is that the simple form of a pit, which in Patella serves to accumulate a secretion which acts as a refractive body, should in Nautilus be glorified and raised to the dignity of an efficient optical apparatus. In all other Mollusca, starting as we may suppose from the follicular or pit-like condition, the eye has proceeded to acquire the form of a closed sac, the cavity of the closed vesicle being then filled partially or completely by a refractive body (lens) secreted by its walls (fig. 14, B). This is the condition attained in most Gastropoda. It presents a striking contrast to the simple Arthropod eye, where, in consequence of the existence of a dense exterior cuticle, the eye does not form a vesicle, and the lens is always part of that cuticle.
 | |
| Fig. 14.—Diagrams of Sections of the Eyes of Mollusca. | |
A, Nautilus (and Patella). B, Gastropod (Limax or Helix). C, Dibranchiate Cephalopod (Oigopsid). Pal, Eyelid (outermost fold). Co, Cornea (second fold). Ir, Iris (third fold). Int 1,2,3,4, Different parts of the integument. l, Deep portion of the lens. |
l1, Outer portion of the lens Co.ep, Ciliary body. R, Retina. N.op, Optic nerve. G.op, Optic ganglion. x, Inner layer of the retina. N.S., Nervous stratum of the retina. (From Balfour, after Grenacher.) |
The development of Nautilus is still entirely unknown. Dr Arthur Willey, during his sojourn in the East Indies, made special efforts to obtain fertilized eggs, both by offering rewards to the native fishermen and collectors and by keeping the living adults in captivity, but without success.
Phylogeny and Classification.—As Nautilus is the only living genus of the Tetrabranchiata, our knowledge of all the rest is based upon the study of their fossil shells. A vast number of species of shell similar in structure to that of Nautilus are known, chiefly from Primary and Secondary formations. These are divided into two sub-orders by differences in the form and structure of the initial chamber. In the Nautiloidea this chamber has the form of an obtuse cone, on the apex of which is a slit-like mark or cicatrix, elongated dorso-ventrally and placed opposite to the blind end of the siphuncle, which indents the front wall of the initial chamber but does not enter its cavity. In the Ammonoidea, on the other hand, the initial chamber is inflated, and is spheroidal, oval or pyriform in shape, with no cicatrix, and separated from the first air-chamber by a constriction. The siphuncle also commences with a dilatation which deeply indents the front wall of the initial chamber, called the protoconch, but does not penetrate into its cavity. Munier-Chalmas has shown that the cavity of the protoconch is traversed by a tubular organ, the “prosiphon,” which does not communicate with the true siphuncle, the place of which it is supposed to take in the early life of the animal. It is generally held, as suggested by Alpheus Hyatt, that the initial chamber of the Nautiloidea corresponds not to the protoconch of the Ammonoids, but to the second chamber of the latter, and that there existed in the young Nautiloids a true initial chamber, a protoconch which was either uncalcified or deciduous. The shell of the living nautilus does not decide this question, as its early stages are unknown, and there is a little vacuity in the centre of the spirally coiled shell which may have been originally occupied by the true protoconch.
The septa in the Nautiloidea are generally concave towards the aperture of the shell, their curvature therefore directed backwards (fig. I); in the Ammonoidea, on the other hand, the convexity is usually towards the aperture, the curvature therefore directed forwards. The lines along which the edges of the septa are united to the shell are known as “sutures,” and these in the Nautiloidea are simply curved or slightly lobed, whereas in the Ammonoidea they are folded in various degrees of complexity; the projections of the suture towards the mouth of the shell are called saddles, those in the opposite direction lobes. The siphuncle in the Nautilus pierces the centres of the septa, and in fossil Nautiloids it is usually central or sub-central. In a few cases it is marginal, and in that case may be external, i.e. ventral, or internal, i.e. dorsal. In Ammonoids the siphuncle is always marginal, and usually external. Its walls in the living Nautilus are strengthened by the deposit of calcareous granules, and in some fossil forms the wall is completely calcified. But this proper calcified wall is quite distinct from calcareous tubes surrounding the siphuncle, which are developed from the septa. In the pearly nautilus each septum is prolonged backwards at the point where it is pierced by the siphuncle, forming a shelly tube somewhat like the neck of a bottle. In many fossil forms these septal necks are continued from the septum from which they arise to the next, so that the siphuncle is enclosed in a complete secondary calcareous tube. In the majority of Nautiloids the septal necks are directed backwards, and they are said to be retrosiphonate. In the majority of the Ammonoids the septal necks are continued forwards from the septa to which they belong, and such forms are termed prosiphonate.
The Tetrabranchiata were most abundant in the Palaeozoic and Mesozoic periods. The Nautiloidea are the most ancient, appearing first in the Upper Cambrian, the genera being most numerous in the Palaeozoic period, and comparatively few surviving into the Secondary. On the other hand, the Ammonoidea are scarce in Palaeozoic formations, being represented in deposits earlier than the Carboniferous only by comparatively simple types, such as Clymenia and Goniatites. In the Secondary period Ammonoids were very abundant, both in genera and species and in individuals, and with few local exceptions none are known to have survived even to the commencement of the Tertiary. In the widest sense the genus Nautilus has existed since the Ordovician (Silurian) period, but the oldest types are not properly to be placed in the same genus as the existing form. Even with this qualification the genus is very ancient, shells very similar to those of the living Nautilus being found in the Upper Cretaceous.
It has been maintained by some zoologists that the Ammonoidea were Dibranchiate, though it would not follow from this that the shell was, therefore, internal. They are, however, generally classed with the Tetrabranchiata, and the absence of all evidence of the possession of an ink-sac is in favour of this view. There can be little doubt that they gave rise to the Dibranchiata.
About 2500 fossil species are included in the Nautiloidea, but only a few species of the genus Nautilus survive. Some of the fossil forms are very large, the shell reaching a length of 2 metres, or 6 ft. 6 in. Of the Ammonoidea more than 5000 species have been described, and some of the coiled forms are 70 cm., or nearly 2 ft. 6 in. in diameter.
Associated with various forms of Ammonoids there have been found peculiar horny or calcified plates, sometimes contained within the body-chamber of the shell, sometimes wholly detached. The most typical form of these structures has been named aptychus. It consists of two bilaterally symmetrical halves, of somewhat semicircular shape, and attached to one another by their straight inner margins, like a pair of doors. In some cases the aptychus is thin and horny, but more often it is thick and calcified, in which case the principal layer has a peculiar cellular structure. The surface may be smooth or sculptured, and one side is usually marked by concentric lines of growth. Another type is similar, except that the two halves are united in the middle line; bodies of this character are called synaptychus; they occur in the body-chamber of species of Scaphites. Another form called anaptychus consists of a thin horny undivided plate which is concentrically striated. This is associated with species of Ammonites and Goniatites.
Many theories have been proposed in explanation of these structures. According to Sir Richard Owen, the aptychus is an operculum developed in a part of the body corresponding to the hood of Nautilus. E. Ray Lankester suggested that the double plate was borne on the surface of the nidamental gland, with the form and sculpturing of which in Nautilus it closely agrees. On this view the aptychus would occur only in females. The most recent view is that these structures could not have been opercula because of their constant position inside the body-chamber, and that they were not external secretions at all, but a calcified internal cartilage situated at the base of the funnel.
Classification of Tetrabranchiata.—Cephalopoda in which the mantle is entirely enclosed by a multilocular siphunculated shell, which may or may not be coiled. Only the last compartment of the shell occupied by the body of the animal. Numerous pedal tentacles around the mouth, which are retractile within sheaths. Halves of the funnel not united. Two pairs of ctenidia, and two pairs of renal tubes without reno-pericardial apertures. Pericardium opens directly to exterior. Cephalic cartilage wholly ventral. Optic vesicles with apertures, without crystalline lens.
Sub-order 1. Nautiloidea.—Initial chamber not inflated, with dorso-ventral cicatrix at extremity.
Fam. 1. Orthoceratidae. Shell straight or slightly curved, with a simple aperture, large terminal chamber and cylindrical siphuncle. Orthoceras, Silurian to Trias. Baltoceras, Silurian.
Fam. 2. Actinoceratidae. Shell straight or slightly curved, with wide siphuncle contracted at level of septa. Actinoceras, Silurian to Carboniferous. Discosorus, Silurian. Huronia, Silurian. Loxoceras, Silurian to Carboniferous.
Fam. 3. Endoceratidae. Shell straight, with wide margina siphuncle, necks produced into tubes fitting into one another. Endoceras, Silurian.
Fam. 4. Gomphoceratidae. Shell globular, straight or arcuate, aperture contracted. Gomphoceras, Silurian. Phragmoceras, Silurian.
Fam. 5. Ascoceratidae. Shell straight, ampulliform, summit truncate, terminal chamber extending nearly whole length of shell ventrally. Ascoceras, Silurian. Glossoceras, Silurian.
Fam. 6. Poterioceratidae. Shell straight or curved, fusiform, aperture simple, siphuncle contracted at septa. Poterioceras, Silurian to Carboniferous. Streptoceras, Silurian.
Fam. 7. Cyrtoceratidae. Shell slightly curved, aperture simple, siphuncle wide, septa approximated. Cyrtoceras, Devonian.
Fam. 8. Lituitidae. Shell coiled in one plane with the terminal part uncoiled, aperture contracted. Lituites, Silurian. Ophidioceras, Silurian.
Fam. 9. Trochoceratidae. Shell helicoidally coiled, dextral or sinistral, the last whorl generally uncoiled. Trochoceras, Devonian. Adelphoceras, Devonian.
Fam. 10. Nautilidae. Shell coiled in one plane, aperture wide and simple, siphuncle central. Nautilus, recent. Trocholites, Silurian. Gyroceras, Silurian to Carboniferous. Hercoceras, Silurian. Ptenoceras, Devonian. Discites, Carboniferous.
Fam. 11. Bactritidae. Shell straight, conical, siphuncle narrow and marginal, necks long, infundibuliform, sutures undulating. Bactrites, Silurian and Devonian.
Sub-order 2. Ammonitoidea,—Initial chamber spheroidal; siphuncle narrow and simple; septa convex towards aperture; sutures complex.
Tribe 1. Retrosiphonata.—Siphuncular necks projecting behind the septa as in Nautiloidea. Sutures form simple undulations. Occur exclusively in Palaeozoic strata from Devonian upwards.
Fam. 1. Goniatitidae. Shell nautiloid, with simple sutures and ventral siphuncle. Goniatites, Devonian and Carboniferous. Anarcestes, Devonian.
Fam. 2. Clymeniidae. Shell nautiloid, with simple sutures, siphuncle dorsal, that is, internal. Clymenia, Upper Devonian.
Tribe 2. Prosiphonata.—Siphuncular necks projecting in front of the septa. Sutures form deeply indented lobes and saddles.
Fam. 1. Arcestidae. Globular and smooth or nearly smooth, with reduced umbilicus, terminal chamber very deep, an aptychus present. Popanoceras, Permian. Cyclolobus, Permian, Arcestes, Trias. Lobites, Trias.
Fam. 2. Tropitidae. Shells globular, but having radiating and tuberculated costae. Thalassoceras, Permian. Tropites, Trias. Sibirites, Trias.
Fam. 3. Ceratitidae. Shells coiled, with a large umbilicus, terminal chamber short, sutures with simple saddles. Trachyceras, Upper Trias. Ceratites, Trias. Dinarites, Trias.
Some genera with helicoidal shells are related to these coiled forms, viz. Cochloceras, Trias; also some straight forms, e.g. Rhab-doccras, Trias.
Fam. 4. Pinacoceratidae. Shell compressed, smooth, terminal chamber short, sutures very complicated, convex. Pinacoceras, Trias.
Fam. 5. Phylloceratidae. Shell coiled, the whorls overlapping each other, sutures formed of numerous lobes and saddles. Phytloceras, Jurassic.
Fam. 6. Lytoceratidae. Shell discoid, whorls loosely united or uncoiled, sutures deeply indented, but with only three saddles and lobes. Lytoceras, Jurassic and Cretaceous. Macroscaphites, Cretaceous. Reunites, Cretaceous. Ptychoeeras, Cretaceous. Turrilites, Cretaceous. Baculites, Cretaceous.
Fam. 7. Ammonitidae. Shell coiled, with narrow whorls which do not embrace one another, aperture simple, a horny anaptychus present. Ammonites, Jurassic. Arietites, Jurassic. Aegoceras, Lias.
Fam. 8. Harpoceratidae. Shell discord and flattened, with a carinated border, aperture provided with lateral projections, a calcareous aptychus, formed of two pieces. Harpoceras, Jurassic. Oppelia, Jurassic. Lissoceras, Jurassic and Cretaceous.
Fam. 9. Amaltheidae. Shell flattened, with a prominent carina continued anteriorly into a rostrum. Amaltheus, Lias. Cardioceras, Jurassic. Schloenbachia, Cretaceous.
Fam. 10. Stephanoceratidae. Shell not carinated, but with radiating costae, which are often bifurcated, aperture often with lateral projections which contract it, aptychus formed of two pieces. Stephanoceras, Morphoceras, Pensphinctes, Peltoceras, Jurassic. Hoplites, Cretaceous. Acanthoceras, Cretaceous. Cosmoceras, Jurassic. Various more or less uncoiled forms are related to this family, viz. Scaphites, Crioceras, Cretaceous.
Order 2. Dibranchiata (= Holosiphona, Acetabulifera)
Characters.—Cephalopoda in which the inflected margins of the epipodia are fused so as to form a complete tubular siphon (fig. 24, i). The circumoral lobes of the fore-foot carry suckers disposed upon them in rows, not tentacles (see figs. 15, 24). There is a single pair of typical ctenidia (fig. 25) acting as gills (hence Dibranchiata), and a single pair of renal organs, opening by apertures right and left of the median anus (fig. 25, r) and by similar internal pores into the pericardial chamber, which consequently does not open directly to the surface as in Nautilus. The oviducts are sometimes paired right and left (Octopoda, Oigopsida), sometimes that of one side only is developed (Myopsida). The sperm-duct is always single except, according to W. Keferstein, in Eledone moschata.
 |
| Fig. 15.—Sepia officinalis, L., about ½ natural size, as seen when dead, the long prehensile arms being withdrawn from the pouches at the side of the head, in which they are carried during life when not actually in use. a. Neck; b, lateral fin of the mantle-sac; c, the eight shorter arms of the fore-foot; d, the two long prehensile arms; e, the eyes. |
A plate-like shell is developed in a closed sac formed by the mantle (figs 20, 21), except in the Octopoda, which have none, and in Spirula (fig. 17, D) and the extinct Belemnitidae, &c., which have a small chambered shell resembling that of Nautilus with or without the addition of plate-like and cylindrical accessory developments (fig. 17, A, C, fig. 19).
The pair of cephalic eyes are highly-developed vesicles with a refractive lens (fig. 33), cornea and lid-folds,—the vesicle being in the embryo, an open sac like that of Nautilus (fig. 34). Osphradia are not present, but cephalic olfactory organs are recognized. One or two pairs of large salivary glands with long ducts are present. An ink-sac formed as a diverticulum of the rectum and opening near the anus is present in all Dibranchiata (fig. 25, t), and has been detected even in the fossil Belemnitidae. Branchial hearts are developed on the two branchial afferent blood-vessels (fig. 28, vc′, vi).
 |
| Fig. 16.—Decapodous Cephalopods. |
A, Cheiroteuthis Veranyi, d’Orb. (from the Mediterranean). B, Thysanoteuthis rhombus, Troschel (from Messina). C, Loligopsis cyclura, Fér. and d’Orb. (from the Atlantic Ocean). |
 |
| Fig. 17.—Internal Shells of Cephalopoda. |
A, Conoteutliis dupiniana, d’Orb. (from the Neocomian of France). B, Shell Sepia orbigniana. Fér. (Mediterranean). C, Shell of Spirulirostra Bellardii, d’Orb. (from the Miocene of Turin). The specimen is cut so as to show in section the chambered shell and the laminated “guard” deposited upon its surface. D, Shell of Splrula laevis, Gray (New Zealand). |
In the Dibranchiata the shell shows various stages of degeneration, culminating in its complete disappearance in Octopus. As in other Mollusca, there is a tendency in Cephalopods for the mantle to extend over the outside of the shell from its edges, and when these secondary mantle-folds entirely cover the shell and meet or fuse together the shell is surrounded by the mantle both externally and internally, and is said to be internal, though it remains always a cuticular structure external to the epidermis. This procebs is generally accompanied by a reduction of the size of the shell in comparison with that of the body, so that the relations of the two are gradually reversed, the body outgrows its house and instead of the mantle being enclosed by the shell, the shell is enclosed by the mantle. The earliest stage of this process is shown in the recent Spirula, though it is perhaps not impossible that in some of the later fossil Ammonoids the shell was becoming more and more internal. The shell of Spirula (fig. 18) is coiled somewhat like that of Nautilus, but the coils are not in contact, the direction of the coil is endogastric or ventral instead of exogastric, and the shell is very much smaller than the body. Like that of Nautilus it is divided by septa and traversed by a siphuncle. The relation of the animal to the terminal chamber is as in Nautilus, but the body extends far beyond the aperture, and folds of the mantle grow up over the shell and cover it everywhere except part of the dorsal and ventral surfaces.
 |
| After Chun, from Lankester’s Treatise an Zoology |
| Fig. 18.—Spirula. |
| A, Dorsal aspect. | pa, Mantle. |
| B, Ventral aspect. | po, Posterior fossa. |
| a, Arms. | sh, Shell. |
| e, Eyes. | te, Tentacular arms. |
| fi, Fins. | td, Terminal pallial disk. |
| fu, Funnel. |
 |
| Fig. 19.—Digram of shell Belemnite (after Phillips). r. Horny pen or “proostracum”: A, conical cavity or “alveolus,” in which the chambered “phragmacone” (p) is contained: g, “guard,” or “rostrum.” |
The next modification in the enclosed shell is the addition to it of secondary deposits of calcareous matter, by the inner surface of the shell-sac. Successive layers are deposited on the posterior part of the original shell, whether coiled or straight, and these layers form a conical mass, which may attain great thickness. A somewhat coiled shell with such a deposit is seen in Spirulirostra (fig. 17, C) of the Miocene. In the next stage of modification secondary secretion forms a long and broad projection of the dorsal lip of the aperture; this is well developed in the belemnites (fig. 19). Thus in these modified shells three parts are to be distinguished: the original septate shell, which has been called the phragmacone; the posterior conical deposit, called the rostrum or guard; and the anterior somewhat flat projection, called the proostracum. In the living Dibranchiata other than Spirula the phragmacone and rostrum have become very rudimentary. The shell of Sepia (fig. 20) consists almost entirely of the proostracum, the little ventral hollow posteriorly representing the phragmacone, and the posterior pointed projection, the rostrum. In the Oigopsida the shell is represented by a proostracum which is no longer calcified by forms a chitinous plume or gladuius, and a similar rudiment occurs in Loliginidac (fig. 21) and Sepiolidae. Lastly, in the Octopoda the shell is represented only by small chitinous rudiments to which the retractor muscles of the head and funnel are attached; these are paired in Octopus, unpaired in other cases as in Cirrhoteuthis.
The early appearance of the sac of the mantle in which the shell is enclosed has led to an erroneous identification of this sac with the primitive shell-sac or shell-gland of the Molluscan embryo. The first appearance of the shell-sac in Dibranchiata is shown in figs. 35, 36. Its formation as an open upgrowth of the centro-dorsal area, and the fact that it appears and disappears without closing in Argonauta and Octopus, was demonstrated by E. Ray Lankester.
 | |
| Fig. 20. | Fig. 21. |
| Fig. 20.—The calcareous internal shell of Sepia officinalis, the so-called cuttle-bone, a, Lateral expansion; b, anterior cancellated region; c, laminated region, the laminae enclosing air. | |
| Fig. 21.—The horny internal shell or gladius or pen of Lohgo. | |
 |
| Fig. 22.—The Argonaut in life. (After Lacaze-Duthiers) Tr. Float: Br.a, anterior arms: Br.p, posterior arms: V, the expanded portion of them, once called the sails; B, the beak; C, the shell; En, the Funnel. |
In Argonauta (the paper nautilus) the female only possesses a shell, in which the body is contained; but this is not homologous with the true shell in other cases; it is a structure sui generis secreted by the expanded arms of the dorsal pair which are closely applied to it on either side (fig. 22).
 |
 |
| Fig. 23.—Head and circumoral processes of the fore-foot
of Onychoteuthis (from Owen). |
Fig. 24.—Male of Ocythoe catenulata, Steenstrup
(Octopus carena, Ver.), showing the hectocotylized
arm. (From Gegenbaur.) |
a, Neck. b, Eye. c, The eight short arms. d, Long prehensile arms, the clavate extremities of which are provided with suckers at e, and with a double row of hooks beyond at f. The temporary conjunction of the arms by means of the suckers enables them to act in combination. |
t1, t2, t3, t4, The first, second, third and fourth arms or processes of the fore-foot. h, The third arm of the right side hectocotylized. x, The apical sac of the hectocotylized arm. y, The filament which issues from the sac when development is complete. i, The siphon. |
Head, Foot, Mantle and Mantle-cavity.—If we now compare the fore-foot of the Dibranchiata with that of Nautilus, we find in the first place a more simple arrangement of its lobes, which are either four or five pairs of tapering processes (called “arms”), arranged in a series around the buccal cone, and a substitution of suckers for tentacles on the surface of these lobes (figs. 15 and 24). The most dorsally placed pair of arms, corresponding to the two sides of the hood of Nautilus, are in reality the most anterior, and are termed the first pair. In the Octopoda there are four pairs of these arms (fig. 38), in the Decapoda five pairs, of which the fourth is greatly elongated (figs. 15, 16). In Sepia, Sepiola and Rossia, each of these long arms is withdrawn into a pouch beside the head, and is only ejected for the purpose of prehension. In Loligo they are completely retractile, very slightly so in the majority of the Oigopsida, and in Rhynchoteuthis they are united to form a beak-like appendage. A gradual reduction of the tentacular arms can be seen in the Decapoda, leading to their total absence in Octopoda; thus in Leachia, Chaunoteuthis and others these arms are reduced to mere stumps. In some Cheiroteuthidae and Cranchiidae the ordinary or sessile arms, especially the dorsal pairs, are reduced. In the Octopoda they are not unfrequently connected by a web, and form an efficient swimming-bell, e.g. in Cirrhoteuthidae and Amphuretidae. The suckers are placed on the adoral surface of the arms, and may be in one, two or four rows, and very numerous. In place of suckers in some genera, e.g. Veranya, we find on certain arms or parts of the arms horny hooks; in other cases a hook rises from the centre of each sucker. The hooks on the long arms of Onychoteuthis are drawn in fig. 23. In various species of Cheiroteuthis the suckers on the tentacular arms are very feeble, but the bottom of the cup is covered by a number of anastomosed epithelial filaments which are used as a fishing-net. The fore-foot, with its apparatus of suckers and hooks, is in the Dibranchiata essentially a prehensile apparatus, though the whole series of arms in the Octopoda serve as swimming organs, and in many (e.g. the common octopus or poulp) the sucker-bearing surface is used as a crawling organ.
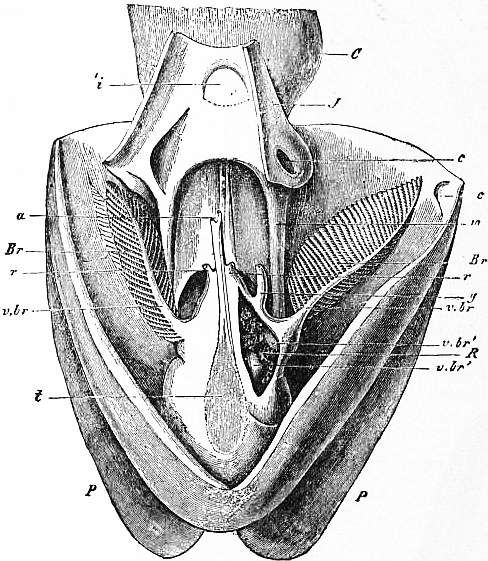 | |
| Fig. 25.—View of the postero-ventral surface of a male Sepia,
obtained by cutting longitudinally the firm mantle-skirt and drawing
the divided halves apart. This figure is strictly comparable with
fig. 4. (From Gegenbaur.) | |
C, The head. J, The mid-foot or siphon, which has been cut open so as to display the valve i. R, The glandular tissue of the left nephridium or renal-sac, which has been cut open (see fig. 29). P, P, The lateral fins of the mantle-skirt. Br, The single pair of branchiae (ctenidia). a, The anus—immediately below it is the opening of the ink-bag. c, Cartilaginous socket in the siphon to receive c′, the cartilaginous knob of the mantle-skirt—the two constituting the “pallial hinge apparatus” characteristic of Decapoda, not found in Octopoda. |
g, The azygos genital papilla and aperture. ′i, Valve of the siphon (possibly the rudimentary hind-foot) m, Muscular band connected with the fore-foot and mid-foot (siphon) and identical with the muscular mass k in fig. 3. r, Renal papillae, carrying the apertures of the nephridia. v.br, Branchial efferent blood-vessel. v br′, Bulbous enlargements of the branchial blood-vessels (see figs 28, 29). t, Ink-bag. |
In the males of the Dibranchiata one of the arms is more or less modified in connexion with the reproductive function, and is called the “hectocotylized arm.” This name is derived from the condition assumed by the arm in those cases in which its modification is carried out to the greatest extent. These cases are those of the Octopods Argonauta argo and Ocythoe catenulata (fig. 24). In the males of these the third arm (on the left side in Argonauta, on the right side in Ocythoe) is found before the breeding season to be represented by a globular sac of integument. This sac bursts, and from it issues an arm larger than its neighbours, having a small sac at its extremity in Ocythoe (fig. 24. x), from which subsequently a long filament issues. Before copulation the male charges this arm with the spermatophores or packets of spermatozoa removed from its generative orifice beneath the mantle-skirt, and during coitus the arm becomes detached and is left adhering to the female by means of its suckers. A new arm is formed at the cicatrix before the next breeding season. The female, being much larger than the male, swims away with the detached arm lodged beneath her mantle-skirt. There, in a way which is not understood, the fertilization of the eggs is effected. Specimens of the female Ocythoë with the detached arm adherent were examined by Cuvier, who mistook the arm for a parasitic worm and gave to it the name Hectocotylus. Accordingly, the correspondingly modified arms of other Cephalopoda are said to be hectocotylized. J.J.S. Steenstrup has determined the hectocotylized condition of one or other of the arms in a number of male Dibranchs as follows:—in all, excepting Argonauta and Ocythoe and Tremoctopus, the modification of the arm is slight, consisting in a small enlargement of part or the whole of the arm, and the obliteration of some of its suckers; in Octopus and Eledone the third right arm is hectocotylized; in Rossia and Sepiola the fourth left arm is hectocotylized along its whole length, and the fourth right arm also in the middle only; in Sepia the fourth left arm is modified at its base only; in Sepioteuthis, the same at its apex; in Loligo, the same also at its apex; in Loliolus, the same along its whole length; in Ommatostrephes, Onychoteuthis and Loligopsis no hectocotylized arm has hitherto been observed. Thus, speaking generally, it is one or both of the fourth pair of short arms which are modified in the Decapoda, of the third pair in the Octopoda. In the pallial cavity are situated one pair of gills in the Dibranchiata (fig. 25), attached dorsally along the whole of their afferent borders. On each side of the branchia is a series of lamellae, least in number in the Octopoda. Each lamella is transversely folded, and the folds are in turn folded, so that the respiratory surface is increased. On the somatic wall of the pallial cavity, between and ventral to the gills, are the following apertures: the anus and opening of the ink-sac, close together in the median line; a pair of apertures of the renal sacs, on either side of the median line; external to the renal orifice, on the left side, the genital aperture in Cirrhoteuthidae and Myopsida. In other Octopoda, and in nearly all the Oigopsida among the Decapoda, the genital ducts are paired in the female, but only the left is developed in the male. The funnel forms a complete tube in the Dibranchiata, and in the majority of the Decapoda, as in Nautilus, it is provided with an internal valve projecting from its somatic surface, which allows water to pass outwards but prevents it passing inwards. The mantle performs rhythmical respiratory movements of expansion and contraction, the water entering between funnel and mantle and passing out through the funnel. In Decapoda the edge of the mantle bears internally on each side a cartilaginous projection which fits into a corresponding depression on the external surface of the funnel; this is called the “resisting apparatus,” and serves to make the union of mantle and funnel firmer during expiration. More powerful expiratory movements are used for sudden retrograde locomotion through the water.
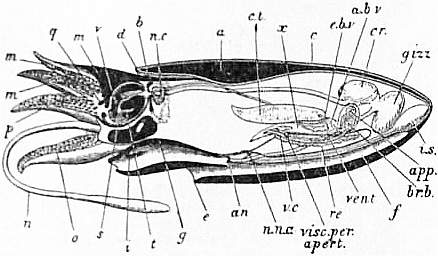 | |
| Fig. 26.—Diagram representing a vertical approximately median
antero-posterior section of Sepia officinalis (from a drawing by A.G.
Bourne). The lettering corresponds with that of fig. 10, with which
this drawing is intended to be compared. | |
a, Shell (here enclosed by a growth of the mantle). b, The nuchal plate (here a cartilage). c, (The reference line should be continued through the black area representing the shell to the outline below it), the integument covering the visceral hump. d, The reflected portion of the mantle-skirt forming the sac which encloses the shell. e, The inferior margin of the mantle-skirt (mouth of the pallial chamber). f, The pallial chamber. g, The vertically cut median portion of the siphon. i, The valve of the siphon. m, The two upper lobes of the fore-foot. n, The long prehensile arms of the same. o, The fifth or lowermost lobe of the fore-foot. p, The third lobe of the fore-foot. q, The buccal membrane. v, The upper beak or jaw. |
s, The lower beak or jaw. t, The lingual ribbon. x, The viscero-pericardial sac. n.c, The nerve-collar. cr, The crop. gizz, The gizzard. an, The anus. c.t, The left ctenidium or gill-plume. vent, Ventricle of the heart. a.b.v, Afferent branchial vessel. e.b.v, Efferent branchial vessel. re, Renal glandular mass. n.n.a, Left nephridial aperture. visc.per.apert, Viscero-pericardial aperture (see fig. 29). br.b, Branchial heart. app, Appendage of the same. i.s, Ink-bag. |
Luminous Organs.—In certain Oigopsida living in deep water, e.g. Histioteuthis, Calliteuthis, Histiopsis, Pterygioteuthis, the surface of the skin bears photogenous organs directed towards the oral extremity. Anatomically these consist of a deeper photogenous layer and a more superficial refracting layer. In some cases, e.g. Pterygioteuthis, they occur even within the mantle-cavity.
Fins.—In the majority of the Decapoda and in the Cirrhoteuthidae, the mantle is produced into lateral symmetrical expansions which have the function of fins. They originate at the aboral extremity where they remain in Spirula (fig. 18). In most other Oigopsida they are terminal, but more dorsal than ventral, e.g. Loligopsis (fig. 16), and there may be two on each side, as in Grimalditeuthis. In other cases they extend laterally along a greater length of the body, as in Sepia (fig. 15). In Ctenopteryx they have a superficial resemblance to the fins of fishes, consisting of a thin membrane supported by a series of muscular rods.
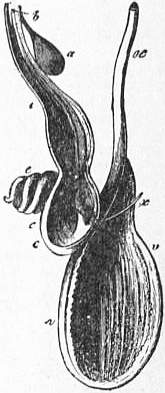 |
| Fig. 27.—Alimentary canal of Loligo sagittata (from Gegenbaur). The buccal mass is omitted. |
oe, Oesophagus. v, The stomach opened longitudinally. x, Probe passed through the pylorus. c, Commencement of the caecum. e, Its spiral portion. i, Intestine. a, Ink-bag. b, Its opening into the rectum. |
Chromatophores.—These are characteristic of the Dibranchiata, apparently absent in Nautilus. They are originally single cells of ectodermic origin which sink below the epidermis and become connected with radiating muscular fibres. The cells are single but multinuclear. Different cells contain pigments of different colours, yellow, brown, red or blue. Each cell in life is in constant tremulous movement; under the influence of nervous excitement the cells are suddenly expanded or contracted, producing blushes of colour and pallor. By reflex action of which the afferent stimulus acts upon the eyes as in fishes, the chromatophores assume a condition which approximates the colour of the animal to that of surrounding objects. In the Decapoda there are also reflecting elements which produce iridescent hues.
Aquiferous Cavities.—In addition to the pockets into which the tentacular arms of Decapoda are retracted, there are in several Dibranchiata cavities in the integument which open to the exterior by special pores but have no communication with the vascular system or other internal cavities of the body. In Ocythoe there are such pores on the back of the head and at the base of the funnel; buccal pouches on the ventral side of the mouth, internal to the arms, occur in some genera, one in Loligo, two in Sepia. In some species of Sepia there are pouches in the mantle.
Alimentary Tube.—The principal differences from Nautilus are the following:—the mandibles are similar in shape, but are chitinous, not calcified. In the radula there are three teeth on each side of the median tooth in each row, except in Gonatus, in which there are only two lateral teeth, and the Cirrhoteuthidae, in which the radula has entirely disappeared. In front of the radula is the so-called tongue, a fleshy projection corresponding to the sub-radular organ of other Mollusca.
In most of the Dibranchiata there are two pairs of salivary glands. In the Decapoda the ducts of the posterior pair unite into a median duct which opens on the surface of the sub-radular organ. The anterior pair is but slightly developed except in the Oigopsida. In the Octopoda there are also two pairs, but the posterior pair, except in Cirrhoteuthis where they are absent, are large and displaced backwards, being situated near the oesophageal proventriculus. Connected with the intestine immediately beyond the pylorus is a thin-walled caecum, spherical in Rossia and Leachia, elongated in Loligo, but usually coiled into a spiral (fig. 27). The hepatic ducts open into the caecum. The liver is developed as a paired gland, more or less fused into one in the adult, but the ducts are always paired. The ducts are covered by a number of glandular follicles forming what is called the pancreas.
The ink-sac, absent in Nautilus, is a rectal caecum developed from its dorsal wall. It is present in all Dibranchiata except Octopus arcticus, O. piscatorum and Cirrhoteuthis. It consists of a deeper part or gland proper and a reservoir. It extends to the posterior extremity of the body in Sepia, but in Octopoda is usually embedded in the surface of the liver. The pigment of the secretion is melanin, and its function is to produce a dense opacity in the water, which conceals the animal.
Vascular System (fig. 28).—The ventricle lies in the pericardial cavity, except in Octopoda where this cavity is much reduced. The auricles, one pair, are contractile expansions of the efferent branchial vessels. The heart gives off an anterior or cephalic and a posterior or abdominal aorta. The vascular system is almost perfect, arteries and veins being united by capillaries. The principal vein is a vena cava passing backwards ventrally from the cephalic region and dividing into two afferent branchial veins, each of which receives a pallial and an abdominal vein. Each of these afferent branchial vessels is enclosed in the cavity of a renal organ and is covered externally by the glandular tissue which forms the excretory part of the “kidney” (fig. 29). Each afferent vessel is expanded into a contractile branchial heart, which is provided with a glandular appendage. The latter corresponds to the glandular masses which are attached to the afferent branchial veins in Nautilus, and to the pericardial glands of other Molluscs.
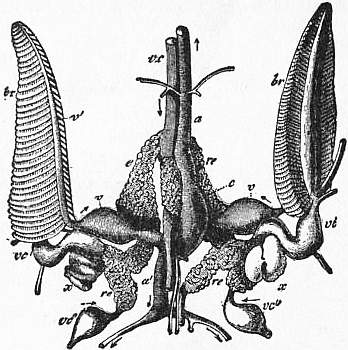 | |
| Fig. 28.—Circulatory and excretory organs of Sepia (from Gegenbaur, after John Hunter). | |
br, Branchiae (ctenidia). c, Ventricle of the heart. a, Anterior artery (aorta). a′, Posterior artery. v, The right and left auricles (enlargements of the efferent branchial veins). v′, Efferent branchial vein on the free face of the gill-plume. v.c, Vena cava. |
vi, vc′, Afferent branchial vessels (branches of the vena cava, see fig. 29). vc″, Abdominal veins. x, Branchial hearts and appendages. re, e, Glandular substance of the nephridia developed on the wall of the great veins on their way to the gills. The arrows indicate the direction of the blood-current. |
 | |
| Fig. 29.—Diagram of the nephridial sacs, and the veins which run
through them, in Sepia officinalis (after Vigelius). The
nephridial sacs are supposed to have their upper walls removed. | |
v.c, Vena cava. r.d.v.c, Right descending branch of the same. r.s.v.c, Left descending branch of the same. v.b.a, Vein from the ink-bag. v.m, Mesenteric vein. v.g, Genital vein. v.a.d, Right abdominal vein. v.a.s, Left abdominal vein. v.p.d, Right pallial vein. v.p.s, Left pallial vein. c.b, Branchial heart. |
x, Appendage of the same. c.v, Capsule of the branchial heart. np, External aperture of the right nephridial sac. y, Reno-pericardial orifice placing the left renal sac or nephridium in communication with the viscero-pericardial sac, the course of which below the nephridial sac is indicated by dotted lines. y′, The similar orifice of the right side. a.r, Glandular renal outgrowths. w.k, Viscero-pericardial sac (dotted outline). |
 | |
| Fig.30. | Fig.31. |
| Figs. 30, 31.—Nerve-centres of Octopus. Figure 30 gives a
view from the dorsal aspect, figure 31 one from the ventral aspect. | |
buc, The buccal mass. ped, Pedal ganglion. opt, Optic ganglion. cer, Cerebral ganglion. pl, Pleural ganglion. |
visc, Visceral ganglion. oes, Oesophagus. f, Foramen in the nerve-mass formed by pedal, pleural and visceral ganglion-pairs, traversed by a blood-vessel. |
 |
| Fig. 32.—Lateral view of the nervous centres and nerves of the right side of Octopus vulgaris (from a drawing by A.G. Bourne). |
|
bg, Buccal ganglion. cer, Cerebral ganglion. ped, Pedal ganglion. pl, Pleural, and visc., visceral region of the pleuro-visceral ganglion. gang. stell, The right stellate ganglion of the mantle connected by a nerve to the pleural portion. n.visc, The right visceral nerve. n.olf, Its (probably) olfactory branches. n.br, Its branchial branches. |
Coelom.—The coelom forms a large sac with a constriction between the anterior or pericardial division and the posterior or genital division, and it is produced into lateral diverticula which contain the branchial hearts; but in the Octopoda the pericardial division is suppressed and the genital division communicates by long ducts with sacs containing the appendages of the branchial hearts. The renal sacs communicate with the pericardium by pores near the external renal apertures; in the Octopoda the reno-pericardial openings are in the capsules of the branchial hearts. The genital ducts pass from the genital coelom to the exterior. They are paired in female Oigopsida and Octopoda except Cirrhoteuthidae, but only the left persists in the males of all Dibranchiata, and in the female Myopsida.
In the oviduct is a glandular enlargement, and in addition to this the females are provided with the so-called nidamental glands which are developed on the somatic wall of the pallial cavity, one on each side of the rectum, except in certain Oigopsida (Enoploteuthis, Cranchia, Leachia) and in the Octopoda, in which these organs are absent. The latter fact is related to the habit of the majority of the Octopoda of guarding or “incubating” their eggs, which have little protective covering. In the other cases the eggs are surrounded by a tough gelatinous elastic material secreted by the nidamental glands.
The vas deferens is at first narrow and convoluted, then dilates into a vesicula seminalis at the end of which is a glandular diverticulum called the prostate. By the vesicula and the prostate the spermatophores are formed. These have a structure similar to those of Nautilus, and in the Octopoda may be as much as 50 mm. in length. Beyond the prostate the duct opens into a large terminal reservoir which has been called Needham’s sac, and in which the spermatophores are stored.
Nervous System and Sense-Organs.—The figures (30, 31, 32) representing the nerve-centres of Octopus serve to exhibit the disposition of these parts in the Dibranchiata. The ganglia are more distinctly swollen than in Nautilus. In Octopus an infra-buccal ganglion-pair are present, corresponding to the buccal ganglion-pair of Gastropoda. In Decapoda a supra-buccal ganglion-pair connected with these are also developed. Instead of the numerous radiating pallial nerves of Nautilus, we have in the Dibranchiata on each side (right and left) a large pleural nerve passing from the pleural portion of the pleuro-visceral ganglion to the mantle, where it enlarges to form the stellate ganglion. From each stellate ganglion nerves radiate to supply the powerful muscles of the mantle-skirt. The two stellate ganglia are connected, except in Sepiola, by a transverse supra-oesophageal commissure, which represents the pallial cords united by a commissure above the intestine in Amphineura. The nerves from the visceral portion of the pleuro-visceral ganglion have the same course as in Nautilus, but no osphradial papilla is present. An enteric nervous system is richly developed in the Dibranchiata, connected with the somatic nervous centres through the buccal ganglia, as in the Arthropoda through the stomato-gastric ganglia, and anastomozing with deep branches of the visceral nerves of the viscero-pleural ganglion-pair. It has been especially described by A. Hancock in Ommatostrephes. Upon the stomach it forms a single large and readily detected gastric ganglion.
 | |
| Fig. 33.—Horizontal section of the eye of Sepia (Myopsid).
(From Gegenbaur, after Hensen.) | |
KK, Cephalic cartilages (see fig. 8). C, Cornea (closed). L, Lens. ci, Ciliary body. Ri, Internal layer of the retina. Re, External layer of the retina. p, Pigment between these. |
o, Optic nerve. go, Optic ganglion. k and k′, Capsular cartilage. ik, Cartilage of the iris. w, White body. ae, Argentine integument. |
 |
| Fig. 34.—Diagrams of sections showing the early stage of development of the eye of Loligo when it is, like the permanent eye of Nautilus and of Patella, an open sac. (From Lankester.) |
| A, First appearance of the eye as a ring-like upgrowth. |
| B, Ingrowth of the ring-like wall so as to form a sac, the primitive optic vesicle of Loligo. |
In the Dibranchiate division of the Cephalopoda the greatest elaboration of the dioptric apparatus of the eye is attained, so that we have in this class the extremes of the two lines of development of the Molluscan eye, those two lines being the punctigerous and the lentigerous. The structure of the Dibranchiate’s eye is shown in section in fig. 14, C, and in fig. 33, and its development in figs. 34 and 37. The open sac which forms the retina of the young Dibranchiate closes up, and constitutes the posterior chamber of the eye, or primitive optic vesicle (fig. 37, A, poc). The lens forms as a structureless growth, secreted by both the internal and external surfaces of the front wall of the optic vesicle (fig. 37, B, l). The integument around the primitive optic vesicle which has sunk below the surface now rises up and forms firstly nearest the axis of the eye the iridian folds (if in B, fig. 37; ik in fig. 33; Ir in fig. 14), and then secondly an outer circular fold grows up like a wall and completely closes over the iridian folds and the axis of the primitive vesicle (fig. 33, C). This covering is transparent, and is the cornea. In the oceanic Decapoda the cornea does not completely close, but leaves a central aperture traversed by the optic axis. These forms are termed Oigopsidae by C. d’Orbigny, whilst the Decapoda with closed cornea are termed Myopsidae. In the Octopoda the cornea is closed, and there is yet another fold thrown over the eye. The skin surrounding the cornea presents a free circular margin, and can be drawn over the surface of the cornea by a sphincter muscle. It thus acts as an adjustable diaphragm, exactly similar in movement to the iris of Vertebrates. Sepia and allied Decapods have a horizontal lower eyelid, that is to say, only one-half of the sphincter-like fold of integument is movable. The statocysts are situated ventrally between the pedal and visceral ganglia, and are entirely enclosed in the cranial cartilage. The cavity of each is continued into a small blind process which is the remnant of the embryonic connexion of the vesicle with the external surface. The sensory epithelium is at the anterior end of the vesicle forming a macula acustica, and in the cavity is a single otolith, partly calcareous and partly organic except in Eledone, in which it is entirely organic. The nerve arises from the cerebral ganglion on each side and passes through the pedal ganglion.
There is no branchial osphradium in the Dibranchiata corresponding to that of Nautilus, but the olfactory organ or rhinophore near the eye is present. In Sepia and the majority of the Dibranchiata it is a simple pit, in some of the Oigopsida it is a projection which may be stalked.
Reproduction and Development.—The modification of one or a pair of the arms in the male for purposes of copulation has already been described. In many genera the sexes differ from one another in other characters also. As a rule the males are more slender or smaller than the females. The maximum degree of sexual dimorphism occurs in Argonauta among the Octopods; in this genus the female may be fifteen times as large as the male, and the peculiar modification of the dorsal arms for the secretion of the shell occurs in the female only, no shell being formed in the male. In most cases the females are much more numerous than the males, but the opposite relation appears to exist in those Octopoda in which the hectocotylus is autotomous, for as many as four hectocotyli have been found in the pallial cavity of a single female. When the hectocotylus is not detached it is usually inserted into the pallial cavity of the female so as to deposit the spermatophores in or near the aperture of the oviduct, but in Sepia and Loligo they are merely deposited on the ventral lobes of the buccal membrane.
The eggs are laid shortly after copulation. In the Octopoda and in Sepia, Sepiola and Rossia, each egg has a separate envelope continued into a long stalk by which it is attached with several others in a cluster. In Argonauta the eggs are carried by the female in the cavity of the shell. In Loligo the eggs are very numerous, and are enclosed in cylindrical transparent gelatinous strings united at one end into a cluster.
The Cephalopoda appear to be the only Invertebrates in which the egg is mesoblastic and telolecithal like that of Vertebrata. This is the result of the large quantity of the yolk, and the position the latter assumes in relation to the blastoderm. In all other Mollusca the segmentation is complete though in some cases very unequal. In the egg of Loligo, which has been chiefly studied (fig. 35), the protoplasmic pole is at the narrower end of the egg, and segmentation is restricted to this end, forming a layer of ectoderm cells. From one part of the periphery of the ectoderm proliferation of cells takes place and gives rise to a layer of scattered nuclei over the whole surface of the yolk. The region of proliferation marks the anal side of the ectoderm, and the layer of nuclei forms the perivitelline membrane. This process must be regarded as equivalent to the first stage of invagination, the yolk being surrounded by hypoblast cells or their nuclei. Later on the same anal edge of the ectoderm forms another cellular layer, the endoderm proper, which forms a continuous sheet below the ectoderm.
The mesoderm also originates at the anal side of the ectoderm and extends in two bands right and left between ectoderm and endoderm. After the mesoderm is thus established, a little vesicle lying upon and open to the yolk is formed from the endoderm, and this vesicle ultimately gives rise to the stomach, the two lobes of the liver and the intestine. The buccal mass and oesophagus arise from a stomodaeal invagination, and the anus is formed later from a short proctodaeal invagination.
 | |
| Fig. 35.—Development of Loligo. | |
1. View of the cleavage of the egg during the first formation of embryonic cells. 2. Lateral view of the egg at a little later stage. a, Limit to which the layer of cleavage-cells has spread over the egg; b, portion of the egg (shaded) as yet uncovered by cleavage-cells; ap, the auto-plasts; kp, cleavage-pole where first cells were formed. 3. Later stage, the limit (a) now extended so as to leave but little of the egg-surface (b) unenclosed. The eyes (d), mouth (e) and mantle-sac (u) have appeared. 4. Later stage, anterior surface, the embryo is becoming nipped off from the yolk-sac (g). 5. View of an embryo similar to (3) from the cleavage-pole or centro-dorsal area. 6. Later stage, posterior surface. 7. Section in a median dorso-ventral and antero-posterior plane of an embryo of the same age as (4). 8. View of the anterior face of an older embryo. 9. View of the posterior face of an embryo of the same age as (8). |
Letters in (3) to (9):—a, lateral fins of the mantle; b, mantle-skirt; c, supra-ocular invagination to form the “white body”; d, the eye; e, the mouth; f1, f2, f3, f4, f5, the five paired processes of the fore-foot; g, rhythmically contractile area of the yolk-sac, which is itself a hernia-like protrusion of the median portion of the fore-foot; h, dotted line showing internal area occupied by yolk (food-material of the egg); k, first rudiment of the epipodia (paired ridges which unite to form the siphon or funnel); l, sac of the radula or lingual ribbon; m, stomach; n, rudiments of the gills (paired ctenidia); o, the otocysts—a pair of invaginations of the surface of the epipodia; p, the optic ganglion; q, the distal portion of the ridges which form the siphon, k being the basal portion of the same structure; r, the vesicle-like rudiment of the intestine formed independently of the parts connected with the mouth, s, k, m, and without invagination; s, rudiment of the salivary glands; t in (7), the shell-sac at an earlier stage open (see fig. 36), now closed up; u, the open shell-sac formed by an uprising ring-like growth of the centro-dorsal area; w in (5), the mantle-skirt commencing to be raised up around the area of the shell-sac. In (7) mes points to the middle cell-layer of the embryo, ep to the outer layer, and h to the deep layer of fusiform cells which separates everywhere the embryo from the yolk or food-material lying within it. |
The external changes of form are as follows:—The mantle is the middle of the embryonic area, and in its centre is the shell-gland, which, however, behaves in a different way from that seen in other Molluscs. Its borders grow inwards and approach each other to form the shell-sac. E. Ray Lankester showed that in Argonauta and other Octopods the shell-sac disappears before it is closed up, but in other forms except Spirula it closes completely and the shell develops within it. The lateral and posterior borders of the embryo form the foot, and these borders grow out into ten or eight lobes which become the arms, and which at first, as seen in fig. 35 (8), are entirely posterior to the mouth. Development actually shows the anterior arms gradually growing round the mouth and uniting in front of it. Between the mantle and the foot are two ridges which form the funnel, and their position shows them to be the epipodia. The otocysts and eyes are formed as invaginations of ectoderm, the former behind the eyes, at the sides of the funnel. All the nerve-centres, cerebral, visceral, pedal and optic, are formed as proliferations of the ectoderm. At the sides of the optic ganglia a pair of ectodermic invaginations are formed, which in the adult become the white bodies of the eyes, surrounding the optic ganglion. These are vestiges of lateral cerebral lobes which degenerate in the course of development.
The coelomic cavity appears as a symmetrical pair of spaces in the mesoderm, right and left of the intestine, and from it grow out the genital ducts and the renal organs. The gonad develops from the wall of the coelom.
 |
| Fig. 36.—Section through aboral end of embryo of Loligo showing shell-sac still open. ep, ectoderm; m, mesoderm; m′, endoderm; shs, shell-sac; y, yolk. |
Phylogeny and Classification.—The order is divided into two sub-orders, Decapoda and Octopoda, by the presence or absence of the tentacular arms. The Decapoda are more adapted for swimming than the Octopoda, the body being usually provided with fins. In the former also there is generally an internal shell of considerable size, often calcined, while in the Octopoda only the merest vestiges of a shell remain. There can be no doubt that the Octopoda were derived from the Decapoda, although from the absence of skeletal structures fossil remains of Octopods are almost entirely unknown. Palaeoctopus, however, occurs in the Cretaceous, while shells of Argonauta do not appear before the Pliocene. The Decapoda are abundantly represented in the Secondary formations by the Belemnitidae, whose shell (fig. 19) consists of a straight conical phragmacone covered posteriorly by a very thick rostrum, and produced anteriorly into a thin long proöstracum which is only occasionally preserved. In certain cases remains of the arms provided with hooks, and of the ink-sac, have been recognized. The Belemnitidae appear first in the Upper Trias, attain their maximum development in the Jurassic rocks, and are not continued into the Tertiary period, though represented in the Eocene by a few allied forms.
There is no difficulty in deriving the typical existing Decapoda from Belemnitidae, and many of the extinct forms may have been directly ancestral. Chitinous “pens” like that of Loligo, however, begin to appear in the Jurassic and Cretaceous rocks, so that in this case as in many others the parent form and the modified form existed contemporaneously, and the latter alone has survived. The oldest shells of the Sepia type are from the Eocene, and it is perhaps possible that the Sepiidae arose separately from the Belemnites.
It is a curious fact that no fossil specimens of the genus Spirula have been found, but this may be due to the fact that it occurs only in deep water. At any rate there is no evidence that the shell of Spirula has lost a rostrum and a proöstracum; its characters must be regarded as primitive, not secondary. In the characters of the protoconch and of the commencement of the siphuncle, the shell of Spirula agrees with that of the Ammonoids, and in both its position is ventral, although in most Ammonoids the shell being exogastric the ventral side is the convex or external, while in Spirula the shell is endogastric and the siphuncle internal. The fact that the shell is not completely enclosed by the mantle is also a primitive character.
With regard to the general morphology of the Cephalopoda, it is difficult to reconcile the existence of two pairs of renal tubes as well as a pair of genital ducts in Nautilus with the view that the original Mollusc was unsegmented and had only one pair of coelomoducts. Considering the great specialization, however, and high degree of organization of the Cephalopods, it is evident that the earliest Nautiloid whose remains are known to us must have had a long evolutionary history behind it, and such metamerism as exists may have been developed in the course of its own history. In the other direction the evidence seems to prove that the Dibranchiata with only two renal ducts have been derived from the Tetrabranchiata.
Suborder 1. Decapoda.—Four pairs of ordinary non-retractile arms which are shorter than the body, and one pair of tentacular arms, situated between the third and fourth normal arms on each side and retractile within special pouches. Suckers pedunculated and provided with horny rings, on the tentacular arms confined usually to the distal extremities. Usually a well-developed internal shell, and lateral fins on the edges of the body. Heart in a coelomic cavity; nidamentary glands usually present.
 | |
| Fig. 37.—Right and left sections through embryos of Loligo. (After Lankester.) | |
A, Same stage as fig. 35 (4). B, Same stage as fig. 35 (8); only the left side of the sections is drawn, and the food-material which occupies the space internal to the membrane ym is omitted. al, Rectum. is, Ink-sac. ep, Outer cell-layer. mes, Middle cell-layer. ym, Deep cell-layer of fusiform cells (yolk-membrane). ng, Optic nerve-ganglion. ot, Otocyst. |
wb, The “white body” of the adult ocular capsule forming as an invagination of the outer cell-layer. mtf, Mantle-skirt. g, Gill. ps, Pen-sac or shell-sac, now closed. dg, Dorsal groove. poc. Primitive optic vesicle, now closed (see fig. 34). l, Lens. r, Retina. soc, Second or anterior optic chamber still open. if, Iridean folds. C, The primitive invagination to form one of the otocysts, as seen in fig. 35 (5) and (6). |
Tribe 1. Oigopsida.—A wide aperture in the cornea. Two oviducts in the female. In fossil genera and Spirula, shell has a multilocular phragmacone with a siphuncle; initial chamber globular and larger than the second chamber. The most ancient forms characterized by the small size of the rostrum and proöstracum, and large size of the phragmacone. In the living genera, except Spirula, the shell is a chitinous gladius.
Fam. 1. Belemnoteuthidae. Extinct; shell with well-developed phragmacone, and rostrum merely a calcareous envelope; siphuncular necks directed backwards as in Nautiloidea; ten equal arms provided with hooks. Phragmoteuthis, Trias. Belemnoteuthis, Jurassic and Cretaceous. Acanthoteuthis, Jurassic.
Fam. 2. Aulacoceratidae. Extinct; phragmacone with widely separated septa; rostrum well developed and claviform. Aulacoceras, Trias. Atractites, Trias and Jurassic. Xiphoteuthis, Lias.
Fam. 3. Belemnitidae. Extinct; phragmacone short with ventral siphuncle, prolonged dorsally into long proöstracum; rostrum large and cylindrical. Belemnites, 350 species from Jurassic and Cretaceous. Diploconus, Upper Jurassic.
Fam. 4. Belopteridae. Extinct; rostrum and phragmacone well developed, phragmacone often curved; initial chamber small. Beloptera, Eocene. Bayanoteuthis, Eocene. Spirulirostra, Miocene.
Fam. 5. Spirulidae. Dorsal and ventral sides of posterior extremity of shell uncovered by mantle; no rostrum or proöstracum; shell calcareous, coiled endogastrically and sipnunculated; fins posterior. Spirula, three living species known, abyssal.
Fam. 6. Ommatostrephidae. Shell internal and chitinous, ending aborally in a little narrow cone; tentacular arms short and thick; suckers with denticulate rings. Ommatostrephes, fins aboral, simple and rhomboidal, British. Ctenopteryx, fins pectinate, as long as the body; Bathyteuthis, fins terminal, rudimentary; tentacular arms, filiform; abyssal. Rhynchoteuthis, tentacular arms united to form a beak-shaped appendage. Symplectoteuthis. Tracheloteuthis. Doridicus. Architeuthis; this is the largest of Cephalopoda, reaching 60 ft. in length including arms.
Fam. 7. Thysanoteuthidae. Arms enlarged, bearing two rows of suckers and filaments; fins triangular, extending whole length of body. Thysanoteuthis, Mediterranean.
Fam. 8. Onychoteuthidae. Fins terminal; tentacular arms long; suckers with hooks. Onychoteuthis, hook-bearing suckers on tentacular arms only. Enoploteuthis, hook-bearing suckers on all the arms. Veranya, body very short, tentacular arms atrophied in the adult, Mediterranean. Chaunoteuthis, body elongated, tentacular arms atrophied. Pterygioteuthis. Ancistroteuthis. Abralia. Teleoteuthis. Lepidoteuthis.
Fam. 9. Gonatidae. Body elongated; fins terminal; radula with only two lateral teeth. Gonatus.
Fam. 10. Cheiroteuthidae. Tentacular arms long, not retractile; resisting apparatus well developed. Cheiroteuthis, suckers along the whole length of the tentacular arms. Doratopsis, body very long and slender with aboral spine, dorsal arms very short. Histioteuthis, six dorsal arms united by membrane, photogenous organs present. Histiopsis, membrane of dorsal arms only half-way up the arms, photogenous organs present. Calliteuthis, no brachial membrane, photogenous organs present. Grimalditeuthis, two fins on each bide, no tentacular arms.
Fam. 11. Cranchiidae. Eight normal arms, very short; eyes prominent; fins small and terminal. Cranchia, body short, purse-shaped, normal arms short, fins entirely aboral. Loligopsis, body elongated, conical, tentacular arms slender. Leachia, tentacular arms absent, funnel without a valve. Taonius, body elongated, normal arms, rather short, eyes pedunculated.
 |
| Fig. 38.—Octopodous Cephalopods. |
|
A, Pinnoctopus cordiformis, Quoy and Gain (from New Zealand). B, Tremoctopus violaceus, Ver. (from the Mediterranean). C, Cranchia scabra, Owen (from the Atlantic Ocean; one of the Decapoda). D, Cirrhoteuthis Mulleri, Esch. (from the Greenland coast). |
Tribe 2. Myopsida.—No aperture in the cornea. Left oviduct only developed in female. Internal shell without a distinct phragmacone, calcified or simply chitinous.
Fam. 1. Sepiidae. Body wide and flat; fins narrow, extending the whole length of the body; shell calcareous and laminated. Belosepia, a rudiment of rostrum and phragmacone present in shell, Eocene. Sepia, shell with a rostrum, British. Sepiella, shell without a rostrum.
Fam. 2. Sepiolidae. Body short, rounded at the aboral end; fins rounded, inserted in middle of body-length; shell chitinous, small or absent. Sepiola, head united to mantle dorsally, British. Rossia, head not united to mantle, British. Stoloteuthis and Inioteuthis, without shell. Heteroteuthis. Euprymna.
Fam. 3. Idiosepiidae. Body elongated, with rudimentary terminal fins; internal shell almost lost. Idiosepius, 1.5 cm. long, Indian Ocean.
Fam. 4. Sepiadariidae. Body short; mantle united to head dorsally; no shell. Sepiadarium, Pacific Ocean. Sepioloidea, Australian.
Fam. 5. Loliginidae. Body elongated and conical; fins extending forward beyond the middle of body-length; shell chitinous, well developed. Loligo, fins triangular, aboral, British. Sepioteuthis, fins rounded, extending along whole of body-length. Loliolus. Loliguncula. The following fossil genera, known only by their gladius and ink-sac, have been placed near Loligo:—Teuthopsis, Beloteuthis and Geoteuthis, Lias; Phylloteuthis, Cretaceous; Plesioteuthis, Jurassic and Cretaceous.
Suborder 2. Octopoda.—Only four pairs of arms, all similar and longer than the body. Body short and rounded aborally. Suckers sessile. Heart not contained in coelom. No nidamentary glands.
 |
| Fig. 39.—Palaeoctopus Newboldi, the oldest Octopod known. From the Cretaceous rocks of Lebanon. (After H. Woodward.) |
Tribe I. Leioglossa.—No radula. Arms united by a complete membrane. Fins on sides of body.
Fam. Cirrhoteuthidae. Tentacular filaments on either side of the suckers. Cirrhoteuthis, pallial sac prominent, fins large, pelagic. Opisthoteuthis, body flattened, with small fins, deep-sea. Vampyroteuthis, four fins. Palaeoctopus, fossil, Cretaceous.
Tribe 2. Trachyglossa.—Radula present. No fins.
Fam. 1. Amphitretidae. Arms united by membrane; funnel attached to mantle, dividing the pallial aperture into two. Amphitretus, pelagic.
Fam. 2. Alloposidae. All arms united by membrane; mantle joined to head by dorsal band and two lateral commissures. Alloposus, pelagic.
Fam. 3. Octopodidae. Arms long and equal, without membrane; hectocotylus not autotomous. No cephalic aquiferous pores. Octopus, two rows of suckers on each arm, British. Eledone, single row of suckers on each arm. Scaeurgus. Pinnoctopus. Cistopus. Japetella.
Fam. 4. Philonexidae. Hectocotylus autotomous; arms unequal in size; aquiferous pores on head and funnel. Tremoctopus, two dorsal pairs of arms united by membrane. Ocythoë, without interbrachial membrane.
Fam. 5. Argonautidae. Hectocotylus autotomous; no interbrachial membrane; extremities of dorsal arms in female expanded and secreting a shell; males very small, without shell. Argonauta.
Literature.—Use has been freely made above of the article by E. Ray Lankester, on Mollusca, in the 9th edition of this Encyclopedia. For the chief modern works, see Bashford Dean, “Notes on Living Nautilus,” Amer. Nat. xxxv., 1901; Arthur Willey, “Contribution to the Natural History of the Pearly Nautilus,” A. Willey’s Zoological Results, pt. vi. (1902); Foord, Cat. Fossil Cephalopoda in British Museum; Alpheus Hyatt, “Fossil Cephalopods of the Museum of Comp. Zoology,” Bull. Mus. Comp. Zool. (Cambridge, U.S., 1868); Jalta, “I Cefalopodi viventi nel golfo di Napoli,” Fauna und Flora des Golfes von Neapel, xxiii. (1896); Joubin, “Céphalopodes de l’atlantique nord,” “Céph. de la Princesse Alice,” Camp. sci. Albert Ier de Monaco, ix. (1895), xxii. (1900); Paul Pelseneer, “Mollusca,” in the Treatise on Zoology, edited by E. Ray Lankester.
↧ Download as ZWI file | Last modified: 11/17/2022 15:23:26 | 33 views
☰ Source: https://oldpedia.org/article/britannica11/Cephalopoda | License: Public domain in the USA. Project Gutenberg License
 ZWI signed:
ZWI signed: KSF
KSF