Maltose
 From Nwe
From Nwe | Maltose[1] | |
|---|---|
 |
|
| Chemical name | 4-O-α-D-Glucopyranosyl-D-glucose |
| Other names | Maltose Malt sugar Maltobiose |
| Chemical formula | C12H22O11 |
| Molecular mass | 342.1162 g/mol |
| CAS number | [69-79-4] |
| Density | 1.54 g/cm3 [2] |
| Solubility | 1.080 g/ml (20 °C) in water[2] |
| Melting point | 102-103 °C (monohydrate) |
| Boiling point | N/A |
| SMILES | OC[C@H]1O[C@H](O[C@H ]2[C@H](O)[C@@H](O)C(O)O [C@@H]2CO)[C@H](O)[C@@H](O)[C@@H]1O |
| Disclaimer and references | |
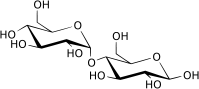
Maltose, or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) linkage. Maltose is not common in food, but can be formed from the digestion of starch, and is heavy in the sugar in malt, the juice of barley and other grains. Maltose is a member of an important biochemical series of glucose chains. The disaccharides maltose, sucrose, and lactose have the same chemical formula, C12H22O11, however, they differ in structure (see structure below).
Maltose can be produced from starch by hydrolysis in the presence of the enzyme diastase. It can be broken down into two glucose molecules by hydrolysis. In living organisms, the enzyme maltase can achieve this very rapidly. In the laboratory, heating with a strong acid for several minutes will produce the same result.
There is another disaccharide that can be made from two glucoses, cellobiose, which differs only in the type of linkage used in the bond. While the difference between these two structures, maltose and cellobiose, is subtle, cellobiose has very different properties and cannot be hydrolized to glucose in the human body. The particular pathways used for these two molecules (some organisms can digest cellobiose) reflects the complex coordination in nature.
Maltose is important in the fermentation of alcohol, as starch is converted to carbohydrates and is readily broken down into glucose molecules with the maltase enzyme present in yeast. When cereals such as barley is malted, it is brought into a condition in which the concentration of maltose has been maximized. Metabolism of maltose by yeast during fermentation then leads to the production of ethanol and carbon dioxide.
Structure
Maltose is a carbohydrate (sugar). Carbohydrates are a class of biological molecules that contain primarily carbon (C) atoms flanked by hydrogen (H) atoms and hydroxyl (OH) groups (H-C-OH). They are named according to the number of carbon atoms they contain, with most sugars having between three and seven carbon atoms termed triose (three carbons), tetrose (four carbons), pentose (five carbons), hexose (six carbons), or heptose (seven carbons).
The single most common monosaccharide is the hexose D-glucose, represented by the formula C6H12O6. In addition to occurring as a free monosaccharide, glucose also occurs in disaccharides, which consist of two monosaccharide units linked covalently. Each disaccharide is formed by a condensation reaction in which there is a loss of hydrogen (H) from one molecule and a hydroxyl group (OH) from the other. The resulting glycosidic bond—those that join a carbohydrate molecule to an alcohol, which may be another carbohydrate—is the characteristic linkage between sugars, whether between two glucose molecules, or between glucose and fructose, and so forth. When two glucose molecules are linked together, such as in maltose, glycosidic bonds form between carbon 1 of the first glucose molecule and carbon 4 of the second glucose molecule. (The carbons of glucose are numbered beginning with the more oxidized end of the molecule, the carbonyl group.)
Three common disaccharides are maltose, sucrose, and lactose. They share the same chemical formula, C12H22O11, but involve different structures. Whereas maltose links two glucose units by an α(1→4) glycosidic linkage, lactose (milk sugar) involves glucose and galactose bonded through a β1-4 glycosidic linkage, and sucrose (common table sugar) consists of a glucose and a fructose joined by a glycosidic bond between carbon atom 1 of the glucose unit and carbon atom 2 of the fructose unit.
Although the disaccharide maltose contains two glucose molecules, it is not the only disaccharide that can be made from two glucoses. When glucose molecules form a glycosidic bond, the linkage will be one of two types, α or β, depending on whether the molecule that bonds its carbon 1 is an α-glucose or β-glucose. An α-linkage with carbon 4 of a second glucose molecule results in maltose, whereas a β-linkage results in cellobiose. As disaccharides, maltose and cellobiose also share the same formula C12H22O11, but they are different compounds with different properties. For example, maltose can be hydrolyzed to its monosaccharides in the human body where as cellobiose cannot. Some organisms have the capacity to break down cellobiose.
The addition of another glucose unit yields maltotriose. Further additions will produce dextrins, also called maltodextrins, and eventually starch.
Function
Maltose is an important intermediate in the digestion of starch. Starch is used by plants as a way to store glucose. After cellulose, starch is the most abundant polysaccharide in plant cells. Animals (and plants) digest starch, converting it to glucose to serve as a source of energy. Maltose can form from this starch when it is broken down, and it in turn can be readily digested into the glucose molecules, the major free sugar circulating in the blood of higher animals, and the preferred fuel of the brain and nervous system. Lu and Sharkey (2006) report that maltose is the predominant form of carbon exported from plant chloroplasts at night, from the breakdown of starch.
Maltose is an interesting compound because of its use in alcohol production. Through a process called fermentation, glucose, maltose, and other sugars are converted to ethanol by yeast cells in the absence of oxygen. Through an analogous process, muscle cells convert glucose into lactic acid to obtain energy while the body operates under anaerobic conditions. Although maltose is uncommon in nature, it can be formed through the breakdown of starch by the enzymes of the mouth.
Maltose, which is one-third as sweet as sucrose, was used to sweeten foods at least as far back as the seventh century by the Chinese (Bender and Bender 2005).
See also
Notes
- ↑ O'Neil, M. J. 2001. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, N.J.: Merck. ISBN 0911910131
- ↑ 2.0 2.1 Biological Engineering Undergraduate Lab, University of Texas. 2002. Material safety data sheet (MSDS) for D-(+)-maltose monohydrate University of Texas as Austin. Retrieved November 18, 2007.
References
ISBN links support NWE through referral fees
- Bender, D. A., and A. E. Bender. 2005. A Dictionary of Food and Nutrition. New York: Oxford University Press. ISBN 0198609612.
- Biological Engineering Undergraduate Lab, University of Texas. 2002. Material safety data sheet (MSDS) for D-(+)-maltose monohydrate University of Texas as Austin. Retrieved November 18, 2007.
- Lu, Y. and T. D. Sharkey. 2006. The importance of maltose in transitory starch breakdown. Plant, Cell and Environment, 29:353-366.
- O'Neil, M. J. 2001. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, N.J.: Merck. ISBN 0911910131.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
↧ Download as ZWI file | Last modified: 02/04/2023 00:16:20 | 38 views
☰ Source: https://www.newworldencyclopedia.org/entry/Maltose | License: CC BY-SA 3.0
 ZWI signed:
ZWI signed:
 KSF
KSF