Tungsten Pentafluoride
 From Handwiki
From Handwiki 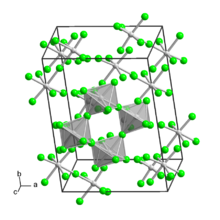
| |
| Names | |
|---|---|
| IUPAC names
Tungsten(V) fluoride
Tungsten pentafluoride | |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
F5W |
| Molar mass | 278.83 g·mol−1 |
| Appearance | yellow solid |
| Density | 5.01 g/cm3 |
| Melting point | 66 °C (151 °F; 339 K) |
| Boiling point | 215.6 °C (420.1 °F; 488.8 K) |
| Hazards | |
| Main hazards | oxidizer, hydrolyzes to release HF |
| Flash point | Non-flammable |
| Related compounds | |
Related compounds
|
TaCl5 NbCl5 MoF5 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tungsten(V) fluoride is an inorganic compound with the formula WF5. It is a hygroscopic yellow solid. Like most pentafluorides, it adopts a tetrameric structure, consisting of [WF5]4 molecules. In this way, each W center achieves octahedral coordination.[1]
Production
Tungsten(V) fluoride is produced by the reaction of tungsten and tungsten hexafluoride:[2]
- W + 5 WF6 → 6 WF5
At room temperature, it disproportionates to the tetra- and hexafluoride:
- 2 WF5 → WF4 + WF6
References
- ↑ Edwards, A. J. (1969). "Crystal Structure of tungsten pentafluoride". J. Chem. Soc. A: 909. doi:10.1039/J19690000909.
- ↑ Schröder, Johann; Grewe, Franz J. (1970). "Darstellung und Eigenschaften von Wolframpentafluorid". Chemische Berichte 103 (5): 1536–46. doi:10.1002/cber.19701030524.
 |
Categories: [Tungsten halides] [Fluorides]
↧ Download as ZWI file | Last modified: 05/28/2025 01:08:32 | 11 views
☰ Source: https://handwiki.org/wiki/Chemistry:Tungsten_pentafluoride | License: CC BY-SA 3.0
✘
ZWI is not signed. [what is this?]

 KSF
KSF