Pentagonal Bipyramidal Molecular Geometry
 From Handwiki
From Handwiki | Pentagonal bipyramidal molecular geometry | |
|---|---|
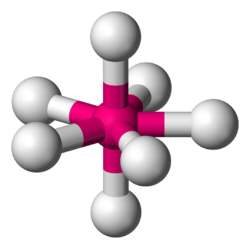 | |
| Examples | IF 7, ZrF 73- |
| Point group | D5h |
| Coordination number | 7 |
| Bond angle(s) | 90°, 72° |
| μ (Polarity) | 0 |
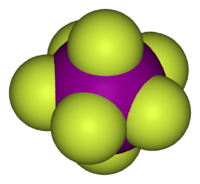
In chemistry, a pentagonal bipyramid is a molecular geometry with one atom at the centre with seven ligands at the corners of a pentagonal bipyramid. A perfect pentagonal bipyramid belongs to the molecular point group D5h.
The pentagonal bipyramid is a case where bond angles surrounding an atom are not identical (see also trigonal bipyramidal molecular geometry).[1] This is one of the three common shapes for heptacoordinate transition metal complexes, along with the capped octahedron and the capped trigonal prism.[2][3]
Pentagonal bipyramids are claimed to be promising coordination geometries for lanthanide-based single-molecule magnets, since (a) they present no extradiagonal crystal field terms, therefore minimising spin mixing, and (b) all of their diagonal terms are in first approximation protected from low-energy vibrations, minimising vibronic coupling.[4]
Examples
- Iodine heptafluoride (IF7) with 7 bonding groups
- Rhenium heptafluoride (ReF7)
- Peroxo chromium(IV) complexes, e.g. [Cr(O2)2(NH3)3] where the peroxo groups occupy four of the planar positions.
- ZrF3−7 and HfF3−7[5][6]
References
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ Roald. Hoffmann; Barbara F. Beier; Earl L. Muetterties; Angelo R. Rossi (1977). "Seven-coordination. A molecular orbital exploration of structure, stereochemistry, and reaction dynamics". Inorganic Chemistry 16 (3): 511–522. doi:10.1021/ic50169a002.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN:0-19-855370-6
- ↑ Duan, Yan; Rosaleny, Lorena E.; Coutinho, Joana T.; Giménez-Santamarina, Silvia; Scheie, Allen; Baldoví, José J.; Cardona-Serra, Salvador; Gaita-Ariño, Alejandro (2022-12-09). "Data-driven design of molecular nanomagnets" (in en). Nature Communications 13 (1): 7626. doi:10.1038/s41467-022-35336-9. ISSN 2041-1723. PMID 36494346. Bibcode: 2022NatCo..13.7626D.
- ↑ Kaupp, Martin (2001). ""Non-VSEPR" Structures and Bonding in d(0) Systems". Angew Chem Int Ed Engl 40 (1): 3534–3565. doi:10.1002/1521-3773(20011001)40:19<3534::AID-ANIE3534>3.0.CO;2-#. PMID 11592184.3.0.CO;2-#&rft_id=info:pmid/11592184&rfr_id=info:sid/en.wikibooks.org:Physics:Pentagonal_bipyramidal_molecular_geometry">
- ↑ Zhenyang Lin; Ian Bytheway (1996). "Stereochemistry of Seven-Coordinate Main Group and d0 Transition Metal Molecules". Inorganic Chemistry 35 (3): 594–603. doi:10.1021/ic950271o.
External links
- [1] – Images of IF7
- 3D Chem – Chemistry, Structures, and 3D Molecules
- IUMSC – Indiana University Molecular Structure Center
 |
Categories: [Stereochemistry] [Molecular geometry]
↧ Download as ZWI file | Last modified: 02/27/2024 03:49:16 | 13 views
☰ Source: https://handwiki.org/wiki/Physics:Pentagonal_bipyramidal_molecular_geometry | License: CC BY-SA 3.0

 KSF
KSF