Plumbane
 From Handwiki
From Handwiki 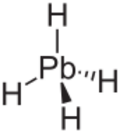
| |
 Lead, Pb Hydrogen, H | |
| Names | |
|---|---|
| IUPAC name
Plumbane
| |
| Other names
lead tetrahydride, tetrahydridolead, lead(IV) hydride, hydrogen plumbide
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider |
|
PubChem CID
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
PbH4 |
| Molar mass | 211.23 g/mol |
| Appearance | Colorless gas |
| Boiling point | −13 °C (9 °F; 260 K) |
| Structure | |
Molecular shape
|
Tetrahedral at the Pb atom |
| Related compounds | |
Related tetrahydride compounds
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
- SizeSet
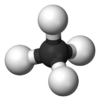
Plumbane is an inorganic chemical compound with the chemical formula PbH4. It is a colorless gas. It is a metal hydride and group 14 hydride composed of lead and hydrogen.[1] Plumbane is not well characterized or well known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom.[2] Derivatives of plumbane include lead tetrafluoride, PbF4, and tetraethyllead, (CH3CH2)4Pb.
History
Until recently, it was uncertain whether plumbane had ever actually been synthesized,[3] although the first reports date back to the 1920s[4] and in 1963, Saalfeld and Svec reported the observation of PbH+4 by mass spectrometry.[5] Plumbane has repeatedly been the subject of Dirac–Hartree–Fock relativistic calculation studies, which investigate the stabilities, geometries, and relative energies of hydrides of the formula MH4 or MH2.[2][6][7]
Properties
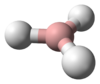
Plumbane is an unstable colorless gas and is the heaviest group IV hydride.[8] Furthermore, plumbane has a tetrahedral (Td) structure with an equilibrium distance between lead and hydrogen of 1.73 Å.[9] By weight percent, the composition of plumbane is 1.91% hydrogen and 98.09% lead. In plumbane, the formal oxidation states of hydrogen and lead are +1 and -4, respectively, because the electronegativity of lead(IV) is higher than that of hydrogen. The stability of metal hydrides with the formula MH4 (M = C–Pb) decreases as the atomic number of M increases.
Preparation
Early studies of PbH4 revealed that the molecule is unstable as compared to its lighter congeners (silane, germane, and stannane).[10] It cannot be made by methods used to synthesize GeH4 or SnH4.
In 1999, plumbane was synthesized from lead(II) nitrate, Pb(NO3)2, and sodium borohydride, NaBH4.[11] A non-nascent mechanism for plumbane synthesis was reported in 2005.[12]
In 2003, Wang and Andrews carefully studied the preparation of PbH4 by laser ablation and additionally identified the infrared (IR) bands.[13]
Congeners
Congeners of plumbane include:
- Methane, CH4
- Silane, SiH4
- Germane, GeH4
- Stannane, SnH4
References
- ↑ Porritt, C. J. (1975). Chem. Ind-London 9: 398.
- ↑ 2.0 2.1 Hein, Thomas A.; Thiel, Walter; Lee, Timothy J. (1993). "Ab initio study of the stability and vibrational spectra of plumbane, methylplumbane, and homologous compounds". The Journal of Physical Chemistry 97 (17): 4381–4385. doi:10.1021/j100119a021.
- ↑ Cotton, F. A.; Wilkinson, G.; Murillo, C. A.; Bochman, M. Advanced Inorganic Chemistry. Wiley: New York, 1999
- ↑ Paneth, Fritz; Nörring, Otto (1920). "Über Bleiwasserstoff". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 53 (9): 1693–1710. doi:10.1002/cber.19200530915. https://zenodo.org/record/1426681.
- ↑ Saalfeld, Fred E.; Svec, Harry J. (1963). "The Mass Spectra of Volatile Hydrides. I. The Monoelemental Hydrides of the Group IVB and VB Elements". Inorganic Chemistry 2: 46–50. doi:10.1021/ic50005a014.
- ↑ Desclaux, J. P.; Pyykko, P. (1974). "Relativistic and non-relativistic Hartree-Fock one-centre expansion calculations for the series CH4 to PbH4 within the spherical approximation". Chemical Physics Letters 29 (4): 534–539. doi:10.1016/0009-2614(74)85085-2. Bibcode: 1974CPL....29..534D.
- ↑ Pyykkö, P.; Desclaux, J. P. (1977). "Dirac–Fock one-centre calculations show (114)H4 to resemble PbH4". Nature 266 (5600): 336–337. doi:10.1038/266336a0. Bibcode: 1977Natur.266..336P.
- ↑ CRC Handbook of Chemistry and Physics Online Edition.
- ↑ Visser, O.; Visscher, L.; Aerts, P. J. C.; Nieuwpoort, W. C. (1992). "Relativistic all-electron molecular Hartree-Fock-Dirac-(Breit) calculations on CH4, SiH4, GeH4, SnH4, PbH4". Theoretica Chimica Acta 81 (6): 405–416. doi:10.1007/BF01134864.
- ↑ Malli, Gulzari L.; Siegert, Martin; Turner, David P. (2004). "Relativistic and electron correlation effects for molecules of heavy elements: Ab initio fully relativistic coupled-cluster calculations for PbH4". International Journal of Quantum Chemistry 99 (6): 940–949. doi:10.1002/qua.20142.
- ↑ Krivtsun, V. M.; Kuritsyn, Y. A.; Snegirev, E. P. (1999). "Observation of IR absorption spectra of the unstable PbH4 molecule". Opt. Spectrosc 86 (5): 686–691. Bibcode: 1999OptSp..86..686K. http://www.isan.troitsk.ru/dms/tdl/publ/1999os_en.pdf. Retrieved 2012-12-31.
- ↑ Zou, Y; Jin, FX; Chen, ZJ; Qiu, DR; Yang, PY (2005). "Non-nascent hydrogen mechanism of plumbane generation". Guang Pu Xue Yu Guang Pu Fen Xi = Guang Pu 25 (10): 1720–3. PMID 16395924.
- ↑ Wang, Xuefeng; Andrews, Lester (2003). "Infrared Spectra of Group 14 Hydrides in Solid Hydrogen: Experimental Observation of PbH4, Pb2H2, and Pb2H4". Journal of the American Chemical Society 125 (21): 6581–6587. doi:10.1021/ja029862l. PMID 12785799.
 |
Categories: [Metal hydrides] [Lead compounds]
↧ Download as ZWI file | Last modified: 08/26/2025 06:36:13 | 6 views
☰ Source: https://handwiki.org/wiki/Chemistry:Plumbane | License: CC BY-SA 3.0


 KSF
KSF