Graphene Foam
 From Handwiki
From Handwiki 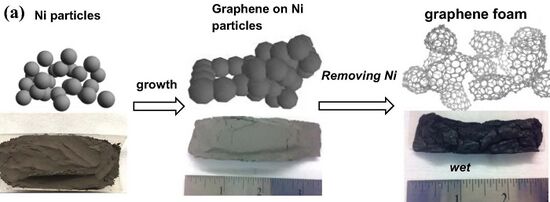

Graphene foam is a solid, open-cell foam made of single-layer sheets of graphene.[1][2] It is a candidate substrate for the electrode of lithium-ion batteries.
Synthesis
The foam can be manufactured using vapor deposition to coat a metal foam, a three-dimensional mesh of metal filaments. The metal is then removed.[1]
Applications
Electrode
A physically flexible battery was created using the foam for electrodes. The anode was made by coating the foam with a lithium-titanium compound (Li4Ti5O12) and the cathode by coating the foam with LiFePO4. Both electrodes were lightweight and their large surface area provided high energy density of 110 Wh/kg, comparable to commercial batteries.[1]
Power density was much greater than a typical battery. At a rate that completely discharged the material in 18 seconds, power delivered was 80 percent of what it produced during an hour-long discharge. Performance remained stable through 500 charge/discharge cycles.[1]
Support
In 2017 researchers used carbon nanotubes to reinforce a foam. The latter material supports 3,000 times its own weight and can return to its original shape when unweighted. Nanotubes, a powdered nickel catalyst and sugar were mixed. Dried pellets of the substance were then compressed in a steel die in the shape of a screw. The nickel was removed, leaving a screw-shaped piece of foam. The nanotubes' outer layers split and bonded with the graphene.[3]
See also
- Aerographene
- Foam
- Lithium-ion battery
References
- ↑ 1.0 1.1 1.2 1.3 Li, Na; Chen, Zongping; Ren, Wencai; Li, Feng; Cheng, Hui-Ming (23 October 2012). "Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates". Proceedings of the National Academy of Sciences 109 (43): 17360–17365. doi:10.1073/pnas.1210072109. PMID 23045691. Bibcode: 2012PNAS..10917360L. + Supplement.
- ↑ Timmer, John (2012). "The fast and the flexible: Graphene foam batteries charge quickly". https://arstechnica.com/science/2012/10/the-fast-and-the-flexible-graphene-foam-batteries-charge-quickly/.
- ↑ Irving, Michael (2017-02-13). ""Rebar graphene" foam supports 3,000 times its own weight". http://newatlas.com/graphene-reinforced-carbon-nanotube-3000-times-weight/47895.
Further reading
- Paronyan, Tereza M.; Thapa, Arjun Kumar; Sherehiy, Andriy; Jasinski, Jacek B.; Jangam, John Samuel Dilip (6 January 2017). "Incommensurate Graphene Foam as a High Capacity Lithium Intercalation Anode". Scientific Reports 7 (1): 39944. doi:10.1038/srep39944. PMID 28059110. Bibcode: 2017NatSR...739944P.
 |
Categories: [Lithium-ion batteries] [Graphene] [Electrodes]
↧ Download as ZWI file | Last modified: 04/05/2024 19:26:12 | 13 views
☰ Source: https://handwiki.org/wiki/Physics:Graphene_foam | License: CC BY-SA 3.0

 KSF
KSF