Lists Of Metalloids
 From Handwiki
From Handwiki This is a list of 194 sources that list elements classified as metalloids. The sources are listed in chronological order. Lists of metalloids differ since there is no rigorous widely accepted definition of metalloid (or its occasional alias, 'semi-metal'). Individual lists share common ground, with variations occurring at the margins. The elements most often regarded as metalloids are boron, silicon, germanium, arsenic, antimony and tellurium.[n 1] Other sources may subtract from this list, add a varying number of other elements, or both.
Overview
| Element | Citations | Frequency | |
|---|---|---|---|
| in n = 194 publications |
194 = 100% | ||
| Arsenic | As | 191.5 | 99% |
| Tellurium | Te | 190.5 | 98% |
| Germanium | Ge | 184.5 | 95% |
| Silicon | Si | 183.5 | 95% |
| Antimony | Sb | 169.5 | 87% |
| Boron | B | 166 | 86% |
| Polonium | Po | 94.5 | 49% |
| Astatine | At | 77 | 40% |
| Selenium | Se | 46 | 24% |
| Aluminium | Al | 18 | 9.3% |
| Carbon | C | 16.5 | 8.5% |
| Bismuth | Bi | 11.5 | 5.9% |
| Phosphorus | P | 10 | 5.2% |
| Beryllium | Be | 7.5 | 3.9% |
| Tin | Sn | 5.5 | 2.8% |
| Sulfur | S | 3 | 1.5% |
| Livermorium | Lv | 3 | 1.5% |
| Iodine | I | 2.5 | 1.3% |
| Flerovium | Fl | 1 | 0.5% |
| Gallium | Ga | 1 | 0.5% |
| Hydrogen | H | 1 | 0.5% |
| Lead | Pb | 1 | 0.5% |
| Moscovium | Mc | 1 | 0.5% |
| Tennessine | Ts | 1 | 0.5% |
Chronological list
This table shows which elements are included in each of 194 different lists of metalloids. A parenthesized symbol indicates an element whose inclusion in a particular metalloid list is qualified in some way by the author(s). The 'citations' rows show how many and what percentage of the authorities consider each element to be a metalloid, with qualified citations counted as one-half.
| Citations as metalloid by element | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Element | Arsenic | Tellurium | Germanium | Silicon | Antimony | Boron | Polonium | Astatine | Selenium | Aluminium | Carbon | Bismuth | Phosphorus | Beryllium | Tin | Sulfur | Livermorium | Iodine | Other | Count | |
| As | Te | Ge | Si | Sb | B | Po | At | Se | Al | C | Bi | P | Be | Sn | S | Lv | I | avg | |||
| Citations (194 = 100%) |
191.5 | 190.5 | 184.5 | 183.5 | 169.5 | 166 | 94.5 | 77 | 46 | 18 | 16.5 | 11.5 | 10 | 7.5 | 5.5 | 3 | 3 | 2.5 | 6 | 7.15 | |
| 99% | 98% | 95% | 95% | 87% | 86% | 49% | 40% | 24% | 9.3% | 8.5% | 5.9% | 5.2% | 3.9% | 2.8% | 1.5% | 1.5% | 1.3% | 3.1% | |||
| Source | Yr | ||||||||||||||||||||
| Simmons[1] | 1947 | As | Te | Sb | Se | 4 | |||||||||||||||
| Szabó & Lakatos[2] | 1954 | As | Te | Ge | Si | Sb | B | Po | At | Al | Be | 10 | |||||||||
| Dull, Metcalfe & Williams[3] | 1958 | As | Te | Ge | Si | Sb | B | Po | At | Al | 9 | ||||||||||
| Frey[4] | 1958 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Johnstone & Miller[5] | 1960 | As | Te | Ge | Si | Sb | B | Se | C | P | 9 | ||||||||||
| Edwards et al.[6] | 1961 | As | Te | Ge | Si | Sb | B | Se | I | 8 | |||||||||||
| Bond[7] | 1962 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Swift & Schaefer[8] | 1962 | As | Ge | Si | Sb | B | Bi | 6 | |||||||||||||
| Hoffman[9] | 1963 | As | Te | Ge | Si | Sb | B | Be | 7 | ||||||||||||
| Nathans[10] | 1963 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Bailar, Moeller & Kleinberg[11] | 1965 | As | Te | Ge | Se | 4 | |||||||||||||||
| Selwood[12] | 1965 | As | Te | Ge | Si | Sb | B | Po | At | Al | Bi | Sn | Ga | 12 | |||||||
| Bassett et al.[13] | 1966 | Te | Ge | Si | Sb | B | Po | Al | Be | 8 | |||||||||||
| Hultgren[14] | 1966 | As | Te | Ge | Si | Sb | Se | C | 7 | ||||||||||||
| Metcalfe, Williams & Castka[15] | 1966 | As | Te | Ge | Si | Sb | B | Po | (Al) | 7.5 | |||||||||||
| Rochow[16] | 1966 | As | Te | Ge | Si | Sb | B | (Po) | (At) | (Se) | (C) | (Bi) | (P) | 9 | |||||||
| Mahan[17] | 1967 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Paul, King & Farinholt[18] | 1967 | As | Te | Ge | Si | Sb | Se | 6 | |||||||||||||
| Siebring[19] | 1967 | As | Te | Ge | Si | Sb | B | Po | Al | 8 | |||||||||||
| Cotton & Lynch[20] | 1968 | As | Te | Ge | Si | Sb | B | At | Se | C | 9 | ||||||||||
| Dunstan[21] | 1968 | As | Te | (Ge) | Sb | Po | Al | Bi | Be | Sn | Pb | 7.5 | |||||||||
| Tyrell & Warren[22] | 1968 | As | Te | (Ge) | Si | (Sb) | B | (Po) | At | (Se) | (Al) | (C) | (P) | (I) | 9.5 | ||||||
| Williams, Embree & DeBey[23] | 1968 | As | Te | Ge | Si | Sb | B | Po | Al | 8 | |||||||||||
| Chedd[24] | 1969 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Hägg[25] | 1969 | As | Te | Ge | Sb | At | Sn | 6 | |||||||||||||
| Holum[26] | 1969 | As | Te | Ge | Si | Sb | B | Po | At | Al | 9 | ||||||||||
| Hunter[27] | 1969 | As | Te | Si | Sb | Se | 5 | ||||||||||||||
| Moody[28] | 1969 | As | Te | Ge | Si | Sb | B | Po | At | Al | Be | 10 | |||||||||
| Dickerson, Gray & Haight[29] | 1970 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Hardwick & Knobler[30] | 1970 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Williams, Embree & DeBay[31] | 1970 | As | Te | Ge | Si | Sb | B | Po | Al | Be | 9 | ||||||||||
| Dickson[32] | 1971 | As | Te | Ge | Si | Sb | Po | 6 | |||||||||||||
| Emsley[33] | 1971 | As | Te | Ge | Sb | 4 | |||||||||||||||
| Nitz & Dhonau[34] | 1971 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Pimentel & Spratley[35] | 1971 | As | Te | Ge | Si | Sb | B | (Po) | (At) | Se | C | 9 | |||||||||
| Barrow[36] | 1972 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Choppin & Johnsen[37] | 1972 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Horvath[38] | 1973 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Pascoe[39] | 1973 | Te | Ge | Si | B | At | Se | C | P | 8 | |||||||||||
| Seager & Stoker[40] | 1973 | As | Te | Ge | Si | Sb | B | Po | At | Al | Be | 10 | |||||||||
| Allen & Keefer[41] | 1974 | As | Te | Ge | Si | Sb | B | At | Se | 8 | |||||||||||
| Andrews[42] | 1974 | As | Te | Si | B | At | 5 | ||||||||||||||
| Day & Johnson[43] | 1974 | As | Te | Ge | Si | Sb | Po | At | 7 | ||||||||||||
| Dickson[44] | 1974 | As | Te | Ge | Si | Sb | Po | At | 7 | ||||||||||||
| Duffy[45] | 1974 | As | Te | Ge | Sb | Se | 5 | ||||||||||||||
| Fuller[46] | 1974 | As | Te | Ge | Si | B | Se | C | 7 | ||||||||||||
| Nordmann[47] | 1974 | As | Te | Ge | Si | B | Po | At | Se | 8 | |||||||||||
| O'Connor[48] | 1974 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Rock & Gerhold[49] | 1974 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Pauling & Pauling[50] | 1975 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Hearst & Ifft[51] | 1976 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Tyler Miller[52] | 1976 | As | Te | Ge | Si | Sb | B | Po | At | Al | H | 10 | |||||||||
| Waser, Trueblood & Knobler[53] | 1976 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Bloomfield[54] | 1977 | As | Te | Ge | Si | Sb | B | Po | At | Al | 9 | ||||||||||
| Ucko[55] | 1977 | As | Te | Ge | Si | Sb | B | Po | At | Al | 9 | ||||||||||
| Hill & Holman[56] | 1978 | As | Te | Ge | Si | B | (C) | 5.5 | |||||||||||||
| Coxon, Fergusson & Phillips[57] | 1980 | As | Te | Ge | Si | (Sb) | B | At | (Be) | 7 | |||||||||||
| Warrena & Geballe[58] | 1981 | As | Te | Si | B | At | Se | C | P | S | 9 | ||||||||||
| Walters[59] | 1982 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Edwards & Sienko[60] | 1983 | As | Te | Ge | Sb | Po | (At) | 5.5 | |||||||||||||
| Holtzclaw, Robinson & Nebergall[61] | 1984 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Boikess & Edelson[62] | 1985 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Peters[63] | 1986 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Hibbert & James[64] | 1987 | As | Te | Ge | Si | Sb | Po | Bi | 7 | ||||||||||||
| Jones et al.[65] | 1987 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| McQuarrie & Rock[66] | 1987 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Wulfsberg[67] | 1987 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Thayer[68] | 1988 | As | Te | Ge | Si | B | P | 6 | |||||||||||||
| Whitten, Gailey & Davis[69] | 1988 | As | Te | Ge | Si | Sb | B | Po | At | Al | 9 | ||||||||||
| Bailar et al.[70] | 1989 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Gill[71] | 1989 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Malone[72] | 1989 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Petrucci[73] | 1989 | As | Te | Ge | Si | Sb | Po | At | 7 | ||||||||||||
| Puddephatt & Monaghan[74] | 1989 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Scott[75] | 1989 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Segal[76] | 1989 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Oxtoby, Nachtrieb & Freeman[77] | 1990 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Atkins & Beran[78] | 1990 | As | Te | Ge | Si | Sb | Po | 6 | |||||||||||||
| Ebbing & Wrighton[79] | 1993 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Zumdahl[80] | 1993 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Birk[81] | 1994 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Smith[82] | 1994 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| AAE[83] | 1996 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Brady & Holum[84] | 1996 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Harrison & de Mora[85] | 1996 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Hook & Post[86] | 1996 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Atkins & Jones[87] | 1997 | As | Te | Ge | Si | Sb | Po | 6 | |||||||||||||
| Dayah[88] | 1997 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Mingos[89] | 1998 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Joesten & Wood[90] | 1999 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Kremer[91] | 1999 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Thompson[92] | 1999 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Umland & Bellama[93] | 1999 | As | Te | Ge | Si | B | At | Se | 7 | ||||||||||||
| Callister[94] | 2000 | As | Te | Ge | Si | B | Se | C | 7 | ||||||||||||
| Enloe[95] | 2000 | As | Te | Si | B | At | 5 | ||||||||||||||
| Mann, Meek & Allen[96] | 2000 | As | Te | Ge | Si | Sb | B | Po | Bi | 8 | |||||||||||
| Phillips, Stozak & Wistrom[97] | 2000 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Ryan[98] | 2000 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Hawkes[99] | 2001 | As | Te | Ge | Sb | Se | Bi | 6 | |||||||||||||
| Lewis & Evans[100] | 2001 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Masterton & Hurley[101] | 2001 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Barrett[102] | 2002 | (As) | (Te) | (Ge) | (Si) | (Sb) | (B) | (Se) | 3.5 | ||||||||||||
| Chang[103] | 2002 | As | Te | Ge | Si | Sb | B | Po | At | Lv | Ts | 10 | |||||||||
| Harding, Johnson & Janes[104] | 2002 | As | Te | Ge | Si | Sb | 5 | ||||||||||||||
| Johnson[105] | 2002 | As | Te | Ge | Si | Sb | At | 6 | |||||||||||||
| Rodgers[106] | 2002 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Szefer[107] | 2002 | As | Te | Ge | Si | Sb | Se | 6 | |||||||||||||
| Woodgate[108] | 2002 | As | Te | Ge | Sb | Al | 5 | ||||||||||||||
| Wright & Welbourn[109] | 2002 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| e-encyclopedia[110] | 2003 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Gupta[111] | 2003 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Hunt[112] | 2003 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Myers[113] | 2003 | As | Te | Ge | Si | Sb | B | At | Se | 8 | |||||||||||
| Williams[114] | 2003 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Atkins[115] | 2004 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Cox[116] | 2004 | As | Te | Ge | Si | Sb | Se | 6 | |||||||||||||
| Gilbert, Kirss & Davies[117] | 2004 | As | Te | Ge | Si | Sb | B | At | Se | 8 | |||||||||||
| Reilly[118] | 2004 | As | Te | Ge | Si | Sb | B | Po | At | Se | 9 | ||||||||||
| Ebbing & Gammon[119] | 2005 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Fry & Page[120] | 2005 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Halliday, Resnick & Walker[121] | 2005 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Holler & Selegue[122] | 2005 | As | Te | Ge | Si | Sb | B | Po | (At) | 7.5 | |||||||||||
| Kotz, Treichel & Weaver[123] | 2005 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Meyer[124] | 2005 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Orchin[125] | 2005 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Swenson[126] | 2005 | As | Te | Ge | Si | Sb | B | Po | At | Se | C | Bi | 11 | ||||||||
| Baird[127] | 2006 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Blei & Odian[128] | 2006 | As | Te | Ge | Si | Sb | Po | At | Lv | 8 | |||||||||||
| Brown & Holme[129] | 2006 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Dashek & Harrison[130] | 2006 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Finch et al.[131] | 2006 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Goldfrank & Flomenbaum[132] | 2006 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Hatt[133] | 2006 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Hérold[134] | 2006 | As | Ge | Si | B | Po | (At) | Se | C | Bi | P | 9.5 | |||||||||
| McMonagle[135] | 2006 | As | Te | Ge | Si | B | Lv | Fl Mc |
8 | ||||||||||||
| Rayner-Canham & Overton[136] | 2006 | As | Te | Ge | Si | B | 5 | ||||||||||||||
| Silberberg[137] | 2006 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Slade[138] | 2006 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Wertheim, Oxlade & Stockley[139] | 2006 | As | Te | Ge | Si | Sb | B | At | Se | 8 | |||||||||||
| Whitley[140] | 2006 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| American Chemical Society[141] | 2007 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Astruc[142] | 2007 | As | Si | B | Se | P | S | 6 | |||||||||||||
| Casper[143] | 2007 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Crystal[144] | 2007 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| DeGraff[145] | 2007 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Joesten, Hogg & Castellion[146] | 2007 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Lewis[147] | 2007 | As | Te | Ge | Si | Sb | B | Po | Se | C | P | S | 11 | ||||||||
| Petty[148] | 2007 | As | Te | Ge | Si | Sb | B | Po | At | Se | C | Bi | P | Sn | 13 | ||||||
| Rösler, Harders & Bäker[149] | 2007 | As | Te | Ge | Si | Sb | B | (Sn) | 6.5 | ||||||||||||
| Saunders[150] | 2007 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Saunders[151] | 2007 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Shipman, Wilson & Tood[152] | 2007 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Bauer, Birk & Sawyer[153] | 2008 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Clugston & Flemming[154] | 2008 | As | Te | Ge | Si | Sb | Se | 6 | |||||||||||||
| Encyclopedia Columbia[155] | 2008 | As | Te | Sb | Se | 4 | |||||||||||||||
| Ham[156] | 2008 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Kelter, Mosher & Scott[157] | 2008 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Masterton & Hurley[158] | 2008 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Merck[159] | 2008 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Nicolaou & Montagnon[160] | 2008 | As | Te | Ge | Si | Sb | B | Po | At | C | 9 | ||||||||||
| Řezanka & Sigler[161] | 2008 | As | Te | Si | B | At | Se | 6 | |||||||||||||
| Tro & Neu[162] | 2008 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Vallero[163] | 2008 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Brown et al.[164] | 2009 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Burrows et al.[165] | 2009 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Castor-Perry[166] | 2009 | As | Te | Ge | Si | Sb | B | Po | At | I | 9 | ||||||||||
| Cracolice & Peters[167] | 2009 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Economou[168] | 2009 | As | Te | Ge | Si | Sb | B | Po | At | Al | 9 | ||||||||||
| Habashi[169] | 2009 | As | Te | Ge | Si | Sb | B | Po | Se | Bi | 9 | ||||||||||
| Hein & Arena[170] | 2009 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Leach[171] | 2009 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Manning[172] | 2009 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| McMurray & Fay[173] | 2009 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Reger, Goode & Ball[174] | 2009 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Schnepp[175] | 2009 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Shubert & Leyba[176] | 2009 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Whitten et al.[177] | 2009 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| Aldinger & Weberruss[178] | 2010 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Banks et al.[179] | 2010 | As | Te | Ge | Si | Sb | B | 6 | |||||||||||||
| Fayer[180] | 2010 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| Gray[181] | 2010 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Groysman[182] | 2010 | As | Te | Ge | Si | Sb | Po | 6 | |||||||||||||
| Halka & Nordstrom[183] | 2010 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Lombi E & Holm PE[184] | 2010 | As | Te | Ge | Si | Sb | B | Po | At | 8 | |||||||||||
| NEST Association[185] | 2010 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| RCCS[186] | 2010 | As | Te | Ge | Si | Sb | B | Po | At | Se | C | Bi | P | Sn | 13 | ||||||
| Senese[187] | 2010 | As | Te | Ge | Si | Sb | (B) | Po | At | (Se) | C | 9 | |||||||||
| Weiner[188] | 2010 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Barbalace[189] | 2011 | As | Te | Ge | Si | Sb | B | Po | 7 | ||||||||||||
| Encyclopædia Britannica Online[190] | 2011 | As | Te | Ge | Si | Sb | B | (Po) | (At) | 7 | |||||||||||
| Helmenstine[191] | 2011 | As | Te | Ge | Si | Sb | B | (Po) | 6.5 | ||||||||||||
| Moore[192] | 2011 | As | Te | Ge | Si | Sb | B | At | 7 | ||||||||||||
| QA International[193] | 2011 | As | Te | Ge | Si | Sb | B | Se | 7 | ||||||||||||
| Element | Arsenic | Tellurium | Germanium | Silicon | Antimony | Boron | Polonium | Astatine | Selenium | Aluminium | Carbon | Bismuth | Phosphorus | Beryllium | Tin | Sulfur | Livermorium | Iodine | Other | ||
- () Parenthesized symbols indicate elements whose inclusion in a particular metalloid list is qualified in some way by the author(s). It is counted as 0.5 citation.
There is an average of 7.15 elements per metalloid list.
Appearance frequency clusters
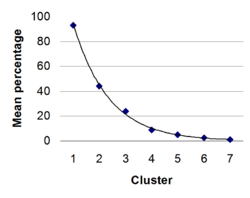
Elements cited in the listed sources (as of August 2011; n = 194) have appearance frequencies that occur in clusters of comparable values. The diamonds in the graph mark the mean appearance frequency of each cluster.
- Cluster 1 (93%): B, Si, Ge, As, Sb, Te
- Cluster 2 (44%): Po, At
- Cluster 3 (24%): Se
- Cluster 4 (9%): C, Al
- Cluster 5 (5%): Be, P, Bi
- Cluster 6 (3%): Sn
- Cluster 7 (1%): H, Ga, S, I, Pb, Fl, Mc, Lv, Ts
The resulting geometric trend line has the formula y = 199.47e−0.7423x and an R2 value of 0.9962.[n 2]
Elements regarded as metalloids
The elements commonly classified as metalloids are boron, silicon, germanium, arsenic, antimony and tellurium.[n 3] The status of polonium and astatine is not settled. Most authors recognise one or the other, or both, as metalloids; Herman, Hoffmann and Ashcroft, on the basis of relativistic modelling, predict astatine will be a monatomic metal.[n 4] One or more of carbon, aluminium, phosphorus, selenium, tin or bismuth, these being periodic table neighbours of the elements commonly classified as metalloids, are sometimes recognised as metalloids.[n 5] Selenium, in particular, is commonly designated as a metalloid in environmental chemistry[n 6] on account of similarities in its aquatic chemistry with that of arsenic and antimony.[n 7] There are fewer references to beryllium, in spite of its periodic table position adjoining the dividing line between metals and nonmetals. Isolated references in the literature can also be found to the categorisation of other elements as metalloids. These elements include: hydrogen, nitrogen,[n 8] sulfur,[n 9] zinc,[n 10] gallium,[n 11] iodine,[n 12] lead,[n 13] and radon[n 14] (citations are for references other than those listed above).
Notes
- ↑ Lack of a rigorous definition:
- Goldsmith RH 1982, 'Metalloids', Journal of Chemical Education, vol. 59, no. 6, pp. 526–527, doi:10.1021/ed059p526
- Hawkes SJ 2001, 'Semimetallicity', Journal of Chemical Education, vol. 78, no. 12, pp. 1686–87, doi:10.1021/ed078p1686
- ↑ The R2 value is a measure of how close a formula fits a set of data points. Values fall between 0.0 and 1.0, with those near 1.0 indicating a good fit.
- ↑ Elements commonly classified as metalloids:
- Goldsmith RH 1982, 'Metalloids', Journal of Chemical Education, vol. 59, no. 6, pp. 526–7 (526), doi:10.1021/ed059p526
- Mann JB, Meek TL & Allen LC 2000, 'Configuration energies of the main group elements', Journal of the American Chemical Society, vol. 122, no. 12, pp. 2780–3 (2783), doi:10.1021ja992866e: Mann et al. refer to these elements as 'the recognized metalloids'.
- Kotz JC, Treichel P & Weaver GC 2009, Chemistry and Chemical Reactivity, 7th ed., Brooks/Cole, Belmont, California, ISBN:1439041318
- ↑ Polonium and astatine:
- Hawkes SJ 2001, 'Semimetallicity?', Journal of Chemical Education, vol. 78, no. 12, pp. 1686–87, doi:10.1021/ed078p1686
- Holt, Rinehart & Wilson c. 2007 'Why polonium and astatine are not metalloids in HRW texts', viewed 8 February 2013
- Hawkes SJ 2010, 'Polonium and astatine are not semimetals', Journal of Chemical Education, vol. 87, no. 8, p. 783, doi:10.1021ed100308w
- Hermann A, Hoffmann R & Ashcroft NW 2013, 'Condensed Astatine: Monatomic and Metallic', Physical Review Letters, vol. 111, pp. 11604–1−11604-5, doi:10.1103/PhysRevLett.111.116404
- Vernon RE 2013, 'Which Elements Are Metalloids?', Journal of Chemical Education, vol. 90, no. 12, pp. 1703–1707, doi:10.1021/ed3008457
- ↑ Carbon, aluminium, phosphorus, selenium, tin, bismuth:
- Rochow EG 1966, The metalloids, DC Heath and Company, Boston, pp. 7–8
- Cobb HM 2012, Dictionary of Metals, ASM International, Materials Park, OH, p. 145, ISBN:9781615039784
- Walker CH 2012, Organic Pollutants: An Ecotoxicological Perspective, 2nd ed., CRC Press, Boca Raton, FL, p. 163, ISBN:9781420062588
- Whiten K, Davis R, Peck L & Stanley G 2014, Chemistry, 10th ed., Brooks/Cole Cengage Learning, Belmont, CA, p. 134, ISBN:9781133610663
- ↑ Selenium as a metalloid in environmental chemistry:
- Meyer JS, Adams WJ, Brix KV, Luoma SM, Mount DR, Stubblefield WA & Wood CM (eds) 2005, Toxicity of dietborne metals to aquatic organisms, Proceedings from the Pellston Workshop on Toxicity of Dietborne Metals to Aquatic Organisms, 27 July – 1 August 2002, Fairmont Hot Springs, British Columbia, Canada, Society of Environmental Toxicology and Chemistry, Pensacola, Florida, p. 284, ISBN:1880611708
- Weiner ER 2013, Applications of Environmental Aquatic Chemistry: A Practical Guide, 3rd ed., CRC Press, Boca Raton, FL, p. 181, ISBN:9781439853320
- ↑ Similarities in the aquatic chemistry of selenium, arsenic, and antimony:
- US Environmental Protection Agency 1988, Ambient aquatic life water quality criteria for antimony (III), draft, Office of Research and Development, Environmental Research Laboratories, Washington, p. 1
- De Zuane J 1997, Handbook of drinking water quality, 2nd ed., John Wiley & Sons, New York, p. 93, ISBN:047128789X
- Uden PC 2005, 'Speciation of selenium,' in R Cornelis, J Caruso, H Crews & K Heumann (eds), Handbook of elemental speciation II: Species in the environment, food, medicine and occupational health, John Wiley & Sons, Chichester, pp. 346–65 (347–8), ISBN:0470855983
- Dev N 2008, 'Modelling selenium fate and transport in Great Salt Lake Wetlands' PhD dissertation, University of Utah, ProQuest, Ann Arbor, Michigan, pp. 2–3, ISBN:054986542X
- ↑ Nitrogen: Rausch MD 1960, 'Cyclopentadienyl compounds of metals and metalloids', Journal of Chemical Education, vol. 37, no. 11, pp. 568–78, doi:10.1021/ed037p568
- ↑ Sulfur:
- Chalmers B 1959, Physical metallurgy, John Wiley & Sons, New York, p. 72
- US Bureau of Naval Personnel 1965, Shipfitter 3 & 2, US Government Printing Office, Washington, p. 26
- ↑ Zinc: Siebring BR 1967, Chemistry, MacMillan, New York, p. 613
- ↑ Gallium: Wiberg N 2001, Inorganic chemistry, Academic Press, San Diego, p. 282, ISBN:0123526515
- ↑ Iodine:
- Friend JN 1953, Man and the chemical elements, 1st ed., Charles Scribner's Sons, New York, p. 68
- Rausch MD 1960, 'Cyclopentadienyl compounds of metals and metalloids', Journal of Chemical Education, vol. 37, no. 11, pp. 568–78, doi:10.1021/ed037p568
- ↑ Lead: Murray JF 1928, 'Cable-sheath corrosion', Electrical World, vol. 92, Dec 29, pp. 1295–7 (1295)
- ↑ Radon:
- Hampel CA & Hawley GG 1966, The encyclopedia of chemistry, 3rd ed., Van Nostrand Reinhold, New York,p. 950
- Stein L 1985, 'New evidence that radon is a metalloid element: ion-exchange reactions of cationic radon', Journal of the Chemical Society, Chemical Communications, vol. 22, pp. 1631–2, doi:10.1039/C39850001631
- Stein L 1987, 'Chemical properties of radon' in PK Hopke (ed.) 1987, Radon and its decay products: Occurrence, properties, and health effects, American Chemical Society, Washington DC, pp. 240–51 (240, 247–8), ISBN:0841210152
References
- ↑ Simmons LM 1947, 'A modification of the periodic table', Journal of Chemical Education, December, pp. 588–591 (589) doi:10.1021/ed024p588
- ↑ Szabó ZG & Lakatos B 1954, 'The new form of the periodic table and new periodic functions', Acta Chimica Academiae Scientiarum Hungaricae, IV 2–4, pp. 129–149 (133)
- ↑ Dull CE, Metcalfe HC & Williams JE 1958, Modern chemistry, Henry Holt and Company, New York, pp. 59–60, 62
- ↑ Frey PR 1958, College chemistry, 2nd ed., Prentice-Hall, Englewood Cliffs, NJ, p. 118
- ↑ Johnstone RT & Miller SE 1960, Occupational diseases and industrial medicine, Saunders, Philadelphia, p. 262
- ↑ Edwards JO, Ellison HR, Luaro CG & Lorand JP 1961, 'Factors which influence the stability of anionic complexes', in S Kirschner, Advances in the chemistry of the coordination compounds: Proceedings of the sixth international conference on coordination chemistry, Macmillan, New York, pp. 230–237 (230)
- ↑ Bond GC 1962, Catalysis by metals, Academic Press, London, p. 8
- ↑ Swift EH & Schaefer WP 1962, Qualitative elemental analysis, WH Freeman, San Francisco, p. 100
- ↑ Hoffman KB 1963, Chemistry for the applied sciences, Prentice-Hall, Englewood Cliffs, NJ, p. 34
- ↑ Nathans MW 1963, Elementary chemistry, Prentice-Hall, Englewood Cliffs, NJ, p. 122
- ↑ Bailar JC, Moeller T & Kleinberg J 1965, University chemistry, DC Heath, Boston, p. 332
- ↑ Selwood PW 1965, General chemistry, 4th ed., Holt, Rinehart and Winston, New York, inside back cover
- ↑ Bassett LG, Bunce SC, Carter AE, Clark HM & Hollinger HB 1966, Principles of chemistry, Prentice-Hall, Englewood Cliffs, NJ, pp. 127, 602
- ↑ Hultgren HH 1966, 'Metalloids, in GL Clark & GG Hawley (eds), The encyclopedia of inorganic chemistry, 2nd ed., Reinhold Publishing, New York, pp. 648–649 (648)
- ↑ Metcalfe HC, Williams JE & Castka JF 1966, Modern chemistry, Hollt, Rinehart and Winston, New York, pp. 466–67
- ↑ Rochow EG 1966, The metalloids, DC Heath, Boston, pp. 3, 7–8
- ↑ Mahan BH 1967, University chemistry, Addison-Wesley, Reading, MA, p. 448
- ↑ Paul MA, King EJ & Farinholt LH 1967, General chemistry, Harcourt, Brace & World, New York, p. 135
- ↑ Siebring BR 1967, Chemistry, Macmillan, New York, p. 70
- ↑ Cotton FA & Lynch LD 1968, Chemistry: An investigative approach, Houghton Mifflin, Boston, p. 226
- ↑ Dunstan S 1968, Principles of chemistry, D Van Nostrand, Princeton, NJ, pp. 310, 409
- ↑ Tyrell LWM & Warren RK 1968, Principles of chemical science: A student's text, Edward Arnold (Publishers), n.p., p. 111
- ↑ Williams AL, Embree HD & DeBey HJ 1968, Introduction to chemistry, Addison-Wesley, Reading MA, inside back cover
- ↑ Chedd G 1969, Half-way elements, Aldus Books, London, p. 24
- ↑ Hägg G 1969, General and inorganic chemistry, John Wiley & Sons, New York, p. 92
- ↑ Holum JR 1969, Introduction to principles of chemistry, John Wiley & Sons, New York, p. 59
- ↑ Hunter D 1969, The diseases of occupations, Little, Brown, & Co., Boston, p. 232
- ↑ Moody BJ 1969, Comparative inorganic chemistry, 2nd ed., Edward Arnold, London, pp. 67–68
- ↑ Dickerson RE, Gray HB & Haight GP 1970, Chemical principles, WA Benjamin, New York, p. 160
- ↑ Hardwick ER & Knobler CM 1979, Chemistry: Man and matter, Xerox College, Waltham, MA, inside back cover
- ↑ Williams AL, Embree HD & DeBay HJ 1970, General chemistry, Addison-Wesley, Reading, MA, p. 55
- ↑ Dickson TR 1971, Introduction to chemistry, John Wiley & Sons, New York, p. 160
- ↑ Emsley J 1971, The inorganic chemistry of the non-metals, Methuen Educational, London, p. 1
- ↑ Nitz OW & Dhonau CA 1971, Chemistry: A brief introduction, Willard Grant, Boston, p. 64
- ↑ Pimentel GC & Spratley RD 1971, Understanding chemistry, Holden-Day, San Francisco, p. 664
- ↑ Barrow GM 1972, General chemistry, Wadsworth, Belmont CA, p. 162
- ↑ Choppin GR & Johnsen RH 1972, Introductory chemistry, Addison-Wesley, Reading MA, p. 346
- ↑ Horvath AL 1973, 'Critical temperature of elements and the periodic system' Journal of Chemical Education, vol 50, no. 5, pp. 335–336 (336)
- ↑ Pascoe KJ 1973, Properties of materials for electrical engineers, John Wiley & Sons, p. 7
- ↑ Seager SL & Stoker HS 1973, Chemistry: A science for today, Scott, Foresman and Company, Glenview, Illinois, p. 58
- ↑ Allen TL & Keefer RM 1974, Chemistry: Experiment and theory, Harper & Row, New York, p. 235
- ↑ Andrews DH 1974, Chemistry: A humanistic view, McGraw-Hill, New York, p. 217
- ↑ Day RA & Johnson RC 1974, General chemistry, Prentice-Hall, Englewood Cliffs, NJ, p. 145
- ↑ Dickson TR 1974, Understanding chemistry: From atoms to attitude, John Wiley & Sons, New York, p. 32
- ↑ Duffy JA 1974, General inorganic chemistry, 2nd ed., Longman, London, p. 53
- ↑ Fuller EC 1974, Chemistry and man's environment, Houghton Mifflin, Boston, p. 186
- ↑ Nordmann J 1974, What is chemistry: a chemical view of nature, Harper & Row, New York, p. 152
- ↑ O'Connor RF 1974, Chemical principles and their biological implications, Hamilton Publishing, Santa Barbara, CA, p. 84
- ↑ Rock PA & Gerhold GA 1974, Chemistry: Principles and applications, WB Saunders, Philadelphia, p. 535
- ↑ Pauling L & Pauling P 1975, Chemistry, WH Freeman, San Francisco, p. 114
- ↑ Hearst JE & Ifft JB 1976, Contemporary chemistry, WH Freeman, San Francisco, p. 104
- ↑ Tyler Miller G 1976, Chemistry: A contemporary approach, Wadsworth, Belmont CA, p. 44
- ↑ Waser J, Trueblood KN & Knobler CM 1976, Chem one, McGraw-Hill, New York, p. 245
- ↑ Bloomfield MM 1977, Chemistry and the living organism, John Wiley & Sons, New York, p. 126
- ↑ Ucko DA 1977, Living chemistry, Academic Press, New York, p. 32
- ↑ Hill GC & Holman JS 1978, Chemistry in context, Thomas Nelson & Sons, Sunbury-on-Thames, Middlesex, p. 27
- ↑ Coxon JM, Fergusson JE & Phillips LF 1980, First year chemistry, Edward Arnold, London, p. 21
- ↑ Warrena JL & Geballe TH 1981, 'Research opportunities in new energy-related materials', Materials Science and Engineering, vol. 50, no.2, Oct, pp. 149–198, p. 161?
- ↑ Walters D 1982, Chemistry, Franklin Watts Science World series, Franklin Watts, London, p. 33
- ↑ Edwards PP & Sienko MJ, 'On the occurrence of metallic character in the periodic table of the elements', Journal of Chemical Education, vol. 60, no. 9, pp. 691–696 (692)
- ↑ Holtzclaw HF, Robinson WR & Nebergall WH 1984, General chemistry, 7th ed., DC Heath, Lexington, p. 193
- ↑ Boikess RS & Edelson E 1985, Chemical principles, 3rd ed., Harper & Row, New York, p. 35
- ↑ Peters EI 1986, Introduction to chemical principles, 4th ed., Saunders College, Philadelphia, p. 105
- ↑ Hibbert DB & James AM 1987, Macmillan dictionary of chemistry, Macmillan, London, p. 300
- ↑ Jones MM, Johnston DO, Netterville JT, Wood JL & Joeston MD 1987, Chemistry & society, 5th ed., Saunders College, Philadelphia, p. 84
- ↑ McQuarrie DA & Rock PA 1987, General chemistry, 3rd ed., WH Freeman, San Francisco, p. 84
- ↑ Wulfsberg G 1987, Principles of descriptive inorganic chemistry, Brooks/Cole, Monterey, CA, p. 201
- ↑ Thayer JS 1988, Organometallic chemistry: An overview, VCH, New York, p. 3
- ↑ Whitten KW, Gailey KD & Davis RE 1988, General chemistry with qualitative analysis, Saunders College, Philadelphia, p. 139
- ↑ Bailar JC, Moeller T, Kleinberg J, Guss CO, Catellion ME & Metz C 1989 Chemistry, 3rd ed., Harcourt Brace Jovanovich, San Diego, p. 742
- ↑ Gill R 1989, Chemical fundamentals of geology, Unwin Hyman, London, p. 292
- ↑ Malone LJ 1989, Basic concepts of chemistry, John Wiley and Sons, New York, p. 135
- ↑ Petrucci RK 1989, General chemistry: Principles and modern applications, 5th ed., Macmillan, New York, p. 284
- ↑ Puddephatt RJ & Monaghan PK 1989, The periodic table of the elements, Clarendon Press, Oxford, p.40
- ↑ Scott A 1989, Molecular machinery: The principles and powers of chemistry, Basil Blackwell, Oxford, p. 18
- ↑ Segal BG 1989, Chemistry: experiment and theory, 2nd ed., Wiley, New York, p. 965
- ↑ Oxtoby DW, Nachtrieb NH & Freeman WA 1990, Chemistry: Science of change, Saunders College, Philadelphia, inside front cover
- ↑ Atkins PW & Beran JA 1990, General chemistry, 2nd ed., Scientific American Books, New York, p. 44
- ↑ Ebbing DD & Wrighton MS 1993, General chemistry, 4th ed., Houghton Mifflin, Boston, p. 58
- ↑ Zumdahl SS 1993, Chemistry, 3rd ed., Lexington MA, p. 327
- ↑ Birk JP 1994, Chemistry, Houghton Mifflin, Boston, inside cover
- ↑ Smith R 1994, Conquering chemistry, 2nd ed., McGraw-Hill, New York, p. 25
- ↑ Academic American Encyclopedia 1996, vol. 13, 'metalloids', Grolier, Danbury, CT, p. 328
- ↑ Brady JE & Holum JR 1996, Chemistry: The study of matter and its changes, John Wiley & Sons, New York, p. 59
- ↑ Harrison RM & de Mora SJ 1996, Introductory chemistry for the environmental sciences, 2nd ed., Cambridge University, Cambridge, p. 150
- ↑ Hook CC & Post R 1996, Chemistry: Concepts and problems, 2nd ed., John Wiley & Sons, New York, p. 297
- ↑ Atkins P & Jones L 1997, Chemistry: Molecules, matter and change, 3rd ed., WH Freeman, New York, p. 15
- ↑ Dayah M 1997, Dynamic Periodic Table, viewed 14 July 2011
- ↑ Mingos DMP 1998, Essential trends in inorganic chemistry, Oxford University, Oxford, p. 202
- ↑ Joesten MD & Wood JL 1999, World of chemistry: Essentials, 2nd ed., Saunders College/Harcourt Brace, Fort Worth, p. 57
- ↑ Kremer P 1999, ChemGlobe – Periodic table of the elements, viewed 14 July 2011
- ↑ Thompson R, 1999, 'Re: What is the metalloid line and where is it located on the Periodic Table?', MadSci Network
- ↑ Umland JB & Bellama JM 1999, General chemistry, 3rd ed., Brooks/Cole, Pacific Grove, inside front cover
- ↑ Callister WD 2000, Materials science and engineering: An introduction, John Wiley & Sons, New York, p. 17
- ↑ Enloe CL 2000, Physical science: What the technology professional needs to know, John Wiley and Sons, New York, p. 93
- ↑ Mann JB, Meek TL and Allen LC 2000, 'Configuration energies of the main group elements', Journal of the American Chemical Society, vol. 122, pp. 2780–2783
- ↑ Phillips JS, Stozak VS & Wistrom C 2000, Chemistry: Concepts and applications, Glencoe/McGraw Hill, Columbus OH, p. 93
- ↑ Ryan L 2000, Advanced chemistry for you, Nelson Thornes, Cheltenham, p. 92
- ↑ Hawkes SJ 2001, 'Semimetallicity', Journal of Chemical Education, vol. 78, no. 12, pp. 1686–1686
- ↑ Lewis R & Evans W 2001, Chemistry, 2nd ed., Palgrave, Basingstoke, Hampshire, p. 212
- ↑ Masterton WL & Hurley N 2001, Chemistry: Principles and reactions, 4th ed., Harcourt College, Fort Worth, inside front cover
- ↑ Barrett J 2002, Atomic structure and periodicity, Wiley-Interscience, Hoboken, NJ, p. 105
- ↑ Chang R 2002, Chemistry, 7th ed., McGraw-Hill, New York, p. 46
- ↑ Harding C, Johnson DA & Janes R 2002, Elements of the p block, Royal Society of Chemistry, Cambridge, p. 210
- ↑ Johnson DA 2002, Metals and chemical change, Royal Society of Chemistry, Cambridge, p. 22
- ↑ Rodgers GE 2002, Descriptive inorganic, coordination, and solid-state chemistry, 2nd ed., Brooks/Cole Thomson, Australia, p. 235
- ↑ Szefer P 2002, Metals, metalloids and radionuclides in the Baltic Sea ecosystem, Elsevier, Amsterdam, p. 14
- ↑ Woodgate S 2002, GEN periodic table: Metals, metalloids, nonmetals, The University of Auckland
- ↑ Wright DA & Welbourn P 2002, Environmental toxicology, Cambridge University, Cambridge, p. 254
- ↑ e-encyclopedia 2003, Dorling Kindersley, London, p. 161
- ↑ Gupta CK 2003, Chemical metallurgy: principles and practice, Wiley-VCH, Weinheim, p. 4
- ↑ Hunt A 2003, Schaum's A-Z: Chemistry, McGraw-Hill, New York, p. 231
- ↑ Myers R 2003, The basics of chemistry, Greenwood, Westport CT, p. 68
- ↑ Williams LD 2003, Chemistry demystified, McGraw-Hill, New York, p. 151
- ↑ Atkins P 2004, Galileo's finger: the ten great ideas of science, Oxford University Press, Oxford, p. 159
- ↑ Cox PA 2004, Inorganic chemistry, 2nd ed., Bios Scientific, London, p. 27
- ↑ Gilbert TR, Kirss RV & Davies G 2004, Chemistry: The science in context, WW Norton, New York, inside front cover
- ↑ Reilly C 2004, The nutritional trace metals, Blackwell, Oxford, p. 5
- ↑ Ebbing DD & Gammon 2005, General chemistry, 8th ed., Houghton Mifflin, Boston, p. 58
- ↑ Fry M & Page E 2005, Catchup chemistry: For the life and medical sciences, Scion, Bloxham, Oxfordshire, p. 14
- ↑ Halliday D, Resnick R & Walker J 2005, Fundamentals of physics, 7th ed., John Wiley & Sons, New York, p. A-15
- ↑ Holler FJ & Selegue JP 2005, The periodic table of comic books, viewed 14 Jul 2011
- ↑ Kotz JC, Treichel P & Weaver GC 2005, Chemistry & chemical reactivity, 6th ed., Brooks Cole, Belmont, CA, p. 80
- ↑ Meyer JS (ed.) 2005, Toxicity of dietborne metals to aquatic organisms, Allen Press/ACG, place p. 282
- ↑ Orchin M 2005, The vocabulary and concepts of organic chemistry, John Wiley and Sons, New York, p. 20
- ↑ Swenson J 2005, 'Classification of noble gases' in Ask a scientist, Chemistry archive
- ↑ Baird C 2006, Chemistry in your life, 2nd ed., WH Freeman, New York, p. 81
- ↑ Blei I & Odian G 2006, General, organic and biochemistry: Connecting chemistry to your life, WH Freeman, New York, inside cover
- ↑ Brown L & Holme T 2006, Chemistry for engineering students, Thomson Brooks/Cole, Belmont, CA, p. 58
- ↑ Dashek WV & Harrison M 2006, Plant cell biology, Science Publishers, Enfield, NH, p. 20
- ↑ Finch J, Sinha R, Singh D & Saika A (eds) 2006, Encyclopedia of science, 3rd ed., Dorling Kindersley, London, p. 32
- ↑ Goldfrank LR & Flomenbaum N 2006, Goldfrank's toxicologic emergencies, McGraw-Hill, New York, p. 187
- ↑ Hatt C 2006, Scientists and their discoveries, Evans Brothers, London, p. 21
- ↑ Hérold A 2006, 'An arrangement of the chemical elements in several classes inside the periodic table according to their common properties', Comptes Rendus Chimie, vol. 9, pp. 148–153
- ↑ McMonagle D 2006, Chemistry: an illustrated guide to science, Infobase Publishing, New York, p. 26
- ↑ Rayner-Canham G & Overton T 2006, Descriptive inorganic chemistry, 4th ed., WH Freeman, New York, p. 29
- ↑ Silberberg MS 2006, Chemistry: The molecular nature of matter and change, 4th ed., McGraw-Hill, New York, p. 55
- ↑ Slade S 2006, Elements and the periodic table, The Rosen Publishing Group, New York, p. 16
- ↑ Wertheim J, Oxlade C & Stockley C 2006, The Usborne illustrated dictionary of chemistry, Usborne, London, p. 51
- ↑ Whitley K 2006, Periodic table: Metals, non-metals, and semi-metals, Chem Professor: Course outline, General chemistry, viewed 14 July 2011
- ↑ American Chemical Society, Interactive periodic table, viewed 14 July 2011
- ↑ Astruc D 2007, Organometallic chemistry and catalysis, Springer, Berlin. p. 312
- ↑ Casper JK 2007, Minerals: Gifts from the earth, Infobase, New York, p. 9
- ↑ Crystal D (ed.) 2007, 'metalloid', in The Penguin Concise Encyclopedia, 3rd ed., Penguin Books, London, p. 599
- ↑ DeGraff J 2007, Understanding and responding to hazardous substances at mine sites in the western United States, Geological Society of America, Boulder, Colordado, p. 26
- ↑ Joesten MD, Hogg JL & Castellion ME 2007, The world of chemistry: essentials, Thommson Higher Education, Belmont, CA, p. 58
- ↑ Lewis RJ 2007, Hawley's condensed chemical dictionary, 15th ed., Wiley-Interscience, New York p. 905
- ↑ Petty MC 2007, Molecular electronics: From principles to practice, vol. 22 of Wiley series in materials for electronic and optoelectronic applications, John Wiley and Sons, New York, p. 25
- ↑ Rösler J, Harders H & Bäker M 2007, Mechanical behaviour of engineering materials: metals, ceramics, polymers and composites, Springer, Berlin, p. 6
- ↑ Saunders N 2007, Exploring atoms and molecules, The Rosen Publishing Group, New York, p. 9
- ↑ Saunders N 2007, Exploring chemical reactions, The Rosen Publishing Group, New York, p. 9
- ↑ Shipman J, Wilson JD & Tood A 2007, An introduction to physical science, Houghton Mifflin, Boston, p. 297
- ↑ Bauer RC, Birk JP & Sawyer DJ 2008, Laboratory inquiry in chemistry, 3rd ed., Brooks/Cole, Belmont, inside back cover
- ↑ Clugston M & Flemming R 2008, Advanced chemistry, Oxford University Press, Oxford, p. 19
- ↑ Encyclopedia Columbia 2008, 'nonmetal', 6th ed., viewed 14 July 2011
- ↑ Ham B 2008, The periodic table, Infobase, New York, p. 66
- ↑ Kelter P, Mosher M & Scott A 2008, Chemistry: The practical science, Media enhanced edition, Houghton Mifflin, Boston, p. 261
- ↑ Masterton Wl & Hurley CN 2008, Chemistry: Principles and reactions, Brooks/Cole Cengage Learning, Belmont, CA, p. 31
- ↑ Merck 2008, Periodic table of the elements, viewed 14 July 2011
- ↑ Nicolaou KC & Montagnon T 2008, Molecules that changed the world: a brief history of the art and science of synthesis and its impact on society, Wiley-VCH, Weinheim, p. 4
- ↑ Řezanka T & Sigler K 2008, 'Biologically active compounds of semi-metals', in Atta-ur-Rahman (ed.), Studies in natural products chemistry, vol. 35, Elsevier, Amsterdam, pp. 835–922 (836)
- ↑ Tro NJ & Neu D 2008, Chemistry in focus: A molecular view of our world, Brooks/Cole, Belmont, CA, p. 75
- ↑ Vallero DA 2008, Fundamentals of air pollution, Academic Press/Elsevier, Burlington MA, p. 200
- ↑ Brown TL, Le May, HE, Bursten BE, Murphy & Woodward 2009, Chemistry: The central science, 11th ed., Pearson Education, Upper Saddle River, NJ, p. 49
- ↑ Burrows A, Holman J, Parsons A, Pilling G & Price G 2009, Chemistry3: Introducing inorganic, organic and physical chemistry, Oxford University, Oxford, p. 1192
- ↑ Castor-Perry S 2009, 'This week in science history – Mendeleev's periodic table', in Science Interviews, The Naked Scientists: Science Radio & Science Podcasts, viewed 14 July 2011
- ↑ Cracolice MS & Peters EI 2009, Introductory chemistry: An active learning approach, Brooks/Cole, Belmont, CA, p. 336
- ↑ Economou EN 2009, The physics of solids: Essentials and beyond, Springer, Heidelberg, p. 59
- ↑ Habashi F 2009, 'Metals: Typical and less typical, transition and inner transition,' Foundations of Chemistry, vol. 12, no. 1, pp. 31–39
- ↑ Hein M & Arena S 2009, Foundations of college chemistry, John Wiley and Sons, alternate 13th ed., New York, p. 49
- ↑ Leach M 2009, 'Periodic table chemical of substances under standard conditions', The chemogenesis webook, viewed 14 July 2011
- ↑ Manning P 2009, Chemical bonds, Infobase, New York, p. 105
- ↑ McMurray J & Fay RC 2009, General chemistry: Atoms first, Prentice Hall, Upper Saddle River, NJ, p. 767
- ↑ Reger DL, Goode SR & Ball DW 2009, Chemistry: Principles & practice, Brooks/Cole, Belmont, CA, p. 56
- ↑ Schnepp R 2009, Hazardous materials: Awareness and operations, Jones & Bartlett Learning, Sudbury, MA, p. 30
- ↑ Shubert D & Leyba J 2009, Chemistry and physics for nurse anesthesia: A student centred approach, Springer, NY, p. 41
- ↑ Whitten KW, Davis RE, Peck ML, Stanley GG 2009, Chemistry, 9th ed. Revised, Brooks/Cole, Belmont, CA, p. 134
- ↑ Aldinger F & Weberruss VA 2010, Advanced ceramics and future materials: An introduction to structures, properties, technologies, methods, Wiley-VCH, Weinheim, p. 49
- ↑ Banks AJ, Bollom MS, Holmes JL, Jacobsen JJ, Kotz JC & Moore JW 2010, Periodic table live!, Division of Chemical Education, viewed 14 July 2011
- ↑ Fayer MD 2010, Absolutely small: How quantum theory explains our everyday world, American Management Association, New York, p. 161
- ↑ Gray T 2010, Metalloids (7), viewed 2 June 2021
- ↑ Groysman A 2009, Corrosion for everybody, Springer, Dordrecht, p. 4
- ↑ Halka M & Nordstrom B 2010, Nonmetals, Infobase, New York, p. xiv
- ↑ Lombi E & Holm PE 2010, 'Metalloids, soil chemistry and the environment', in TP Jahn (ed.), MIPS and their role in the exchange of metalloids, Landes Bioscience, Austin< TX, 33–46(33)
- ↑ National Earth Science Teachers Association 2010, 'Metals, nonmetals, & metalloids', Windows to the universe, viewed 14 July 2011
- ↑ Research Centre for Computational Science 2010, The periodic table of the elements, Okazaki Research Facilities, National Institutes of Natural Sciences, Aichi, Japan, viewed 14 July 2011
- ↑ Senese FA 2010, Metals, nonmetals and metalloids, viewed 14 July 2011
- ↑ Weiner ER 2010, Applications of environmental aquatic chemistry: A practical guide, 2nd ed., CRC Press, Boca Raton, Florida, p. 109
- ↑ Barbalace KL 2011, Periodic table of elements, viewed 14 July 2011
- ↑ "Metalloid". Encyclopædia Britannica Online. 2011. http://www.britannica.com/EBchecked/topic/377645/metalloid. Retrieved 14 July 2011.
- ↑ Helmenstine AM 2011, Metalloids or semimetals: Properties of element groups, viewed 14 July 2011
- ↑ Moore JT 2011, The periodic table: metals, nonmetals, and metalloids, dummies.com, viewed 14 July 2011
- ↑ QA International 2011, Merriam-Webster Visual dictionary online, viewed 14 July 2011
 |
Categories: [Lists of chemical elements]
↧ Download as ZWI file | Last modified: 07/23/2024 20:34:02 | 10 views
☰ Source: https://handwiki.org/wiki/Chemistry:Lists_of_metalloids | License: CC BY-SA 3.0

 KSF
KSF